Abstract
Purpose
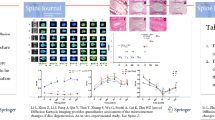
To assess the feasibility of diffusion kurtosis imaging (DKI) for diagnosing lumbar intervertebral disk degeneration (IDD) and to compare the potential of DKI and T2* mapping in the diagnosis of early IDD.
Methods
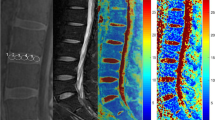
Sagittal T2WI, DKI, and T2* mapping were performed in 75 subjects with 375 lumbar intervertebral disks at a 3.0-T MRI. DKI-related parameters including mean kurtosis (MK), mean diffusivity (MD), fractional anisotropy (FA), and T2* values were calculated for each disk which was segmented into three regions: nucleus pulposus (NP), anterior annulus fibrosus (AAF), and posterior annulus fibrosus (PAF).
Results
MK and FA were positively correlated with Pfirrmann grade (all P < 0.001). MD and T2* were negatively correlated with Pfirrmann grade (all P < 0.001) except for T2* value of AAF (r = 0.087, P > 0.05). MK and FA values increased, while MD and T2* values decreased with age. No statistical significance was found between men and women (P > 0.05). Cephalic lumbar disks (L1/L2 and L2/L3) got lower MK and FA values than caudal lumbar disks (L4/L5 and L5/S1) (all P < 0.05), while cephalic lumbar disks got higher MD value than caudal lumbar disks (all P < 0.05). ROC analysis demonstrated that MK, MD, and FA showed significantly higher diagnostic accuracies than T2*, especially in NP and PAF.
Conclusions
DKI can be used to assess human lumbar IDD. And DKI was more sensitive to the quantitative detection of early lumbar IDD than T2* mapping.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.







Similar content being viewed by others
References
Nilsson M, Lagerstrand K, Kasperska I, Brisby H, Hebelka H (2016) Axial loading during MRI influences T2-mapping values of lumbar discs: a feasibility study on patients with low back pain. Eur Spine J 25:2856–2863
Messner A, Stelzeneder D, Trattnig S et al (2017) Does T2 mapping of the posterior annulus fibrosus indicate the presence of lumbar intervertebral disc herniation? A 3.0 Tesla magnetic resonance study. Eur Spine J 26:877–883
Rajasekaran S, Naresh-Babu J, Murugan S (2007) Review of postcontrast MRI studies on diffusion of human lumbar discs. J Magn Reson Imaging 25:410–418
Newell N, Little JP, Christou A, Adams MA, Adam CJ, Masouros SD (2017) Biomechanics of the human intervertebral disc: a review of testing techniques and results. J Mech Behav Biomed Mater 69:420–434
Zhang XJ, Yang L, Gao F, Yuan ZG, Wang GB (2015) Comparison of T1ρ and T2* relaxation mapping in patients with different grades of disc degeneration at 3T MR. Med Sci Monit 21:1934–1941
Detiger SE, Holewijn RM, Hoogendoorn RJ, Royen BJ, Smit TH (2015) MRI T2* mapping correlates with biochemistry and histology in intervertebral disc degeneration in a large animal model. Eur Spine J 24:1935–1943
Kolf AK, Hesper T, Schleich C, Hosalkar H, Bittersohl B (2016) T2* mapping of ovine intervertebral discs: normative data for cervical and lumbar spine. J Orthop Res 34:717–724
Alkalay RN, Burstein D, Westin CF, Meier D, Hackney DB (2015) MR diffusion is sensitive to mechanical loading in human intervertebral disks ex vivo. J Magn Reson Imaging 41:654–664
Jensen JH, Helpern JA (2010) MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 23:698–710
Arab A, Wojna-Pelczar A, Khairnar A, Szabo N, Ruda-Kucerova J (2018) Principles of diffusion kurtosis imaging and its role in early diagnosis of neurodegenerative disorders. Brain Res Bull 139:91–98
Jensen JH, Helpern JA, Ramani A, Lu HZ, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53:1432–1440
Li L, Zhu WZ, Chen WW, Fang JC, Li J (2017) The study of the intervertebral disc microstructure in matured rats with diffusion kurtosis imaging. Magn Reson Imaging 42:101–106
Falk Delgado A, Nilsson M, Westen DV, Delgado AF (2018) Glioma grade discrimination with mr diffusion kurtosis imaging: a meta-analysis of diagnostic accuracy. Radiology 287:119–127
Das SK, Yang DJ, Wang JL, Zhang C, Yang HF (2017) Non-Gaussian diffusion imaging for malignant and benign pulmonary nodule differentiation: a preliminary study. Acta Radiol 58:19–26
Jiang JX, Tang ZH, Zhong YF, Su JY, Wu FY (2017) Diffusion kurtosis imaging for differentiating between the benign and malignant sinonasal lesions. J Magn Reson Imaging 45:1446–1454
Yin JZ, Sun HZ, Wang ZY, Ni HY, Sun PZ (2018) Diffusion kurtosis imaging of acute infarction: comparison with routine diffusion and follow-up MR imaging. Radiology 287:651–657
Xiao Z, Tang Z, Qiang J, Qian W, Tang W (2018) Differentiation of olfactory neuroblastomas from nasal squamous cell carcinomas using MR diffusion kurtosis imaging and dynamic contrast-enhanced MRI. J Magn Reson Imaging 47:354–361
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26:1873–1878
Lazar M, Jensen JH, Xuan L, Helpern JH (2008) Estimation of the orientation distribution function from diffusional kurtosis imaging. Magn Reson Med 60:774–781
Lotz JC, Haughton V, Boden SD, An HS, Marinelli NL (2012) New treatment and imaging strategies in degeneration disease of the intervertebral disks. Radiology 264:6–19
Urban JP, Winlove CP (2007) Pathophysiology of the intervertebral disc and the challenges for MRI. J Magn Reson Imaging 25:419–432
Kim KW, Chung HN, Ha KY, Lee JS, Kim YY (2009) Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J 9:658–666
Takatalo J, Karppinen J, Niinimäki J, Taimela S, Tervonen O (2012) Association of modic changes, Schmorl’s nodes, spondylolytic defects, high-intensity zone lesions, disc herniations, and radial tears with low back symptom severity among young Finnish adults. Spine 37:1231–1239
Siemionow K, An H, Masuda K, Andersson GB, Cs-Szabo G (2011) The effects of age, sex, ethnicity, and spinal level on the rate of intervertebral disc degeneration: a review of 1712 intervertebral discs. Spine (Phila Pa 1976) 36:1333–1339
Wang YX, Griffith JF (2010) Effect of menopause on lumbar disc degeneration: potential etiology. Radiology 257:318–320
Gübitz R, Lange T, Gosheger G, Heindel W, Schulte TL (2018) Influence of age, BMI, gender and lumbar level on T1ρ magnetic resonance imaging of lumbar discs in healthy asymptomatic adults. Rofo 190:144–151
Oh CH, Yoon SH (2017) Whole spine disc degeneration survey according to the ages and sex using Pfirrmann disc degeneration grades. Korean J Spine 14:148–154
Zhang W, Ma XH, Wang Y, Zhao J, Li SL (2014) Assessment of apparent diffusion coefficient in lumbar intervertebral disc degeneration. Eur Spine J 23:1830–1836
Acknowledgements
This study was supported by the grants from National Natural Science Foundation of China (No. 81871332) and Hubei Key Laboratory of Medical Information Analysis and Tumor Diagnosis & Treatment (No. PJS140011708).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeng, F., Zha, Y., Li, L. et al. A comparative study of diffusion kurtosis imaging and T2* mapping in quantitative detection of lumbar intervertebral disk degeneration. Eur Spine J 28, 2169–2178 (2019). https://doi.org/10.1007/s00586-019-06007-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-06007-z