Abstract
Purpose
Herniated nucleus pulposus has been considered to induce an adaptive immune response. Antigen recognition by antigen-presenting-cells (APCs) represents an important step within manifestation of an adaptive immune response. Macrophages have been assumed to function as APC, while importance of plasmacytoid dendritic cells for initiation of an immune response directed towards herniated nucleus pulposus has never been examined. The aim of the present study was to assess importance of plasmacytoid dendritic cells for initiation of immune response directed towards herniated discs.
Methods
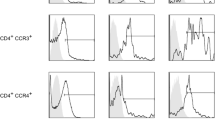
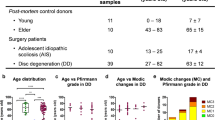
Fifteen patients with true sequestrations and three patients with subligamentous sequestrations underwent surgery after their neurological examinations. Disc material was harvested, weighted and digested for 90 min. Separated single cells were counted, stained for plasmacytoid dendritic cells (CD123+CD4+), macrophages (CD14+CD11c+) and memory T cells (CD4+CD45RO+) and analysed by flow cytometry. Both patient groups were compared in cell proportions. Furthermore, patients with true sequestrations (TRUE patients) were subdivided into subgroups based on severity of muscle weakness and results in straight leg raising (SLR) test. Subgroups were compared in cell proportions.
Results
Plasmacytoid dendritic cells and memory T cells infiltrated true sequestrations stronger than the subligamentous sequestration and plasmacytoid dendritic cells predominated over macrophages in true sequestrations. Highest proportions of plasmacytoid dendritic cells were detected in infiltrates of patients having true sequestrations, severe muscle weakness and negative result in SLR test.
Conclusions
The findings of the present study indicate that plasmacytoid dendritic cells are involved in initiation of an immune response directed towards herniated nucleus pulposus, while macrophages may reinforce the manifested immune response and mediate disc resorption.





Similar content being viewed by others
References
Mixter WJ, Barr J (1934) Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med 211:210–215
Rydevik B, Brown MD, Lundborg G (1984) Pathoanatomy and pathophysiology of nerve root compression. Spine 9:7–15
Garfin SR, Rydevik BL, Brown RA (1991) Compressive neuropathy of spinal nerve roots. A mechanical or biological problem? Spine 16:162–166
Olmarker K, Larsson K (1998) Tumor necrosis factorα and nucleus pulposus-induced nerve root injury. Spine 23:2538–2544
Geiss A, Larsson K, Junevik K et al (2009) Autologous nucleus pulposus primes T cells to develop into interleukin-4-producing effector cells: an experimental study on the autoimmune properties of nucleus pulposus. J Orthop Res 27:97–103
Bobechko WP, Hirsch C (1965) Auto-immune response to nucleus pulposus in the rabbit. J Bone Joint Surg 47:574–580
Gertzbein SD, Trait JH, Devlin SR (1977) The stimulation of lymphocytes by nucleus pulposus in patients with degenerative disc disease of the lumbar spine. Clin Orthop Relat Res 123:149–154
Ahn SH, Ahn MW, Byun WM (2000) Effect of the Transligamentous extension of lumbar disc herniation on their regression and the clinical outcome of sciatica. Spine 25:475–480
Brock M, Patt S, Mayer HM (1992) The form and structure of the extruded disc. Spine 17:1457–1461
Abbas AK, Lichtman AH, Pillai S (2012) Cellular and molecular immunology. Saunders/Elsevier, Philadelphia
Saal JS, Franson RC, Dobrow R et al (1990) High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine 15:674–678
Kobayashi S, Yoshizawa H, Yamada S (2004) Pathology of lumbar nerve root compression Part 1: intraradicular inflammatory changes induced by mechanical compression. J Orthop Res 22:170–179
Cui JG, Holmin S, Mathiesen T et al (2000) Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain 88:239–248
Boos N, Rieder R, Schade V et al (1995) The diagnostic accuracy of magnetic resonance imaging, work perception, and psychosocial factors in identifying symptomatic disc herniations. Spine 20:2613–2625
Virri J, Grönblad M, Seitsalo S et al (2001) Comparison of the prevalence of inflammatory cells in subtypes of disc herniations and associations with straight leg raising. Spine 26:2311–2315
Grönblad M, Virri J, Seitsalo S et al (2000) Inflammatory cells, motor weakness, and straight leg raising in transligamentous disc herniations. Spine 25:2803–2807
Shamji MF, Setton LA, Jarvis W et al (2010) Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum 62:1974–1982
Rothoerl R, Woertgen C, Holzschuh M et al (1998) Macrophage tissue infiltration, clinical symptoms, and signs in patients with lumbar disc herniation. A clinicopathological study on 179 patients. Acta Neurochir 140:1245–1248
Kawaguchi S, Yamashita T, Yokogushi K et al (2001) Immunophenotypic analysis of the inflammatory infiltrates in herniated intervertebral discs. Spine 26:1209–1214
Colonna M, Trinchieri G, Liu YJ (2004) Plasmacytoid dendritic cells in immunity. Nat Immunol 5:1219–1226
Andersson GB, Weinstein JN (1996) Disc herniation. Spine 21(Suppl 24):1S
Masaryk TJ, Ross JS, Modic MT et al (1988) High-resolution MR imaging of sequestered lumbar inter-vertebral disks. Am J Roentgenol 150:1155–1162
Frankel HL, Hancock DO, Hyslop G et al (1969) The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I Paraplegia 7:179–192
Balaji VR, Chin KF, Tucker S (2014) Recovery of severe motor deficit secondary to herniated lumbar disc prolapse: is surgical intervention important? A systematic review. Eur Spine J 23:1968–1977
Hornsby PJ (1980) Regulation of cytochrome P-450 supported 11-hydroxylation of deoxycortisol by steroids, oxygen, and antioxidants in adrenocortical cell cultures. J Biol Chem 255:4020–4027
Facchetti F, Vermi W, Mason D, Colonna M (2003) The plasmacytoid monocytes/interferon producing cells. Virchows Arch 443:703–717
Bland M (2000) An introduction to medical statistics, 3rd edn. Oxford University Press, Oxford
Risbud MV, Shapiro IM (2014) Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 10:44–56
Buckwalter JA (1995) Aging and degeneration of the human intervertebral disc. Spine 20:1307–1314
Holm S, Nachemson A (1988) Nutrition of the intervertebral disc: acute effects of cigarette smoking. An experimental animal study. Upsala J Med 93:91–99
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737
Kapsenberg ML (2003) Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 3:984–993
Liu YJ (2005) IPC: professional type I interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol 23:275–306
Swiecki M, Colonna M (2015) The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 15:471–485
Gilliet M, Cao W, Liu YJ (2008) Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol 8:594–606
Lövgren T, Eloranta M-L, Bave U, Alm GV, Rönnblom L (2004) Induction of Interferon- production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 50:1861–1872
Villadangos JA, Young L (2008) Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 29:352–361
Stirling A, Worthington T, Rafiq M, Lambert PA, Elliot TSJ (2001) Association between sciatica and Propionibacterium acnes. Lancet 357:2014–2025
Gank R, Rao PJ, Phan K, Mobbs RJ (2015) Can bacterial infection by low virulent organisms be a plausible cause for symptomatic disc degeneration? Spine 40:E587–E592
Lotz JC, Chin JR (2000) Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine 25:1477–1483
Bibby SRS, Urban JPG (2004) Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J 13:695–701
Satoh K, Konno S, Nishiyama K et al (1999) Presence and distribution of antigen-antibody complexes in the herniated nucleus pulposus. Spine 24:1980–1984
Klawitter M, Hakozaki M, Kobayashi H et al (2014) Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells. Eur Spine J 23:1878–1891
Cella M, Jarrossay D, Facchetti F et al (1999) Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nature Med 5:919–923
Bondue B, Wittamer V, Parmentier M (2011) Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev 22:331–338
Doita M, Kanatani T, Ozaki T, Matsui N, Kurosaka M, Yoshiya S (2001) Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine 26:1522–1527
Ikeda T, Nakamura T, Kikuchi T, Umeda S, Senda H, Takagi K (1996) Pathomechanism of spontaneous regression of the herniated lumbar disc: histologic and immunohistochemical study. J Spinal Disord 9:136–140
Campbell DJ, Koch MA (2011) Phenotypical and functional specialization of FOXP3+regulatory T cells. Nat Rev Immunol 11:119–130
Rissoan MC, Soumelis V, Kadowaki N et al (1999) Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183–1186
Agak GW, Qin M, Nobe J et al (2014) Propionibacterium acnes induces an IL-17 response in acne vulgaris that is regulated by vitamin A and vitamin D. J Invest Dermatol 134:366–373
Shin MS, Lee N, Kang I (2011) Effector T cell subsets in systemic lupus erythematosus: update focusing in TH17 cells. Curr Opin Rheumatol 23:444–448
Park JB, Chang H, Kim YS (2002) The pattern of interleukin-12 and T-helper types 1 and 2 cytokine expression in herniated lumbar disc tissue. Spine 27:2125–2128
Gilfillan AM, Tkaczyk C (2006) Integrated signalling pathways for mast-cell activation. Nat Rev Immunol 6:218–230
Garcia-Faroldi G, Rönnberg E, Orro A et al (2013) ADAMTS: novel proteases expressed by activated mast cells. Biol Chem 394:291–305
Geiss A, Larsson K, Rydevik B et al (2007) Autoimmune properties of nucleus pulposus.An experimental study in pigs. Spine 32:168–173
Richards JO, Treisman J, Garlie N et al (2012) Flow cytometry assessment of residual melanoma cells in tumor-infiltrating lymphocytes cultures. Cytometry A 81:374–381
Waymouth C (1974) To disaggregate or not to disaggregate injury and cell disaggregation, transient or permanent? In Vitro 10:97–111
Abuzakouk M, Feighery C, O’Farrelly C (1996) Collagenase and Dispase enzymes disrupt lymphocyte surface molecules. J Immunol Methods 194:211–216
Lasfargues EY, Moore DH (1971) A method for the continuous cultivation of mammary epithelium. In Vitro 7:21–25
Delaney TJ, Rowlingson JC, Carron H, Butler A (1980) Epidural steroid effects on nerves and meninges. Anesth Analg 59:610–614
Olmarker K, Byröd G, Cornefjord M et al (1994) Effects of Methylprednisolone on nucleus pulposus-induced nerve root injury. Spine 19:1803–1808
Acknowledgments
The work was supported by grants from the German Research Foundation (GE 1232/5-1) and the University Hospital of Cologne, Department of Orthopaedics and Traumatology, D-50937 Köln, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Geiss, A., Sobottke, R., Delank, K.S. et al. Plasmacytoid dendritic cells and memory T cells infiltrate true sequestrations stronger than subligamentous sequestrations: evidence from flow cytometric analysis of disc infiltrates. Eur Spine J 25, 1417–1427 (2016). https://doi.org/10.1007/s00586-015-4325-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-4325-z