Abstract
 Subarachnoid pleural fistula (SPF) is a type of cerebrospinal fluid (CSF) fistula that can arise as a complication following transthoracic resection of intervertebral disc herniation in the thoracic spine. It is an abnormal communication between the subarachnoid and pleural space. Negative intrapleural pressure promotes CSF leak due to a suction effect into the pleural cavity, with little chance of spontaneous closure. Due to the risk of severe complications with CSF leak into the thoracic cavity, early diagnosis and treatment are mandatory. However, management can be challenging. We report a case of a 72-year-old woman who underwent anterior thoracic surgery to treat thoracic myelopathy caused by an ossified intradural disc herniation. The postoperative period was complicated by a subarachnoidal pleural fistula. We describe our successful treatment of this using noninvasive positive pressure ventilation and lumbar CSF drainage and review other methods reported in the literature.
Subarachnoid pleural fistula (SPF) is a type of cerebrospinal fluid (CSF) fistula that can arise as a complication following transthoracic resection of intervertebral disc herniation in the thoracic spine. It is an abnormal communication between the subarachnoid and pleural space. Negative intrapleural pressure promotes CSF leak due to a suction effect into the pleural cavity, with little chance of spontaneous closure. Due to the risk of severe complications with CSF leak into the thoracic cavity, early diagnosis and treatment are mandatory. However, management can be challenging. We report a case of a 72-year-old woman who underwent anterior thoracic surgery to treat thoracic myelopathy caused by an ossified intradural disc herniation. The postoperative period was complicated by a subarachnoidal pleural fistula. We describe our successful treatment of this using noninvasive positive pressure ventilation and lumbar CSF drainage and review other methods reported in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case report
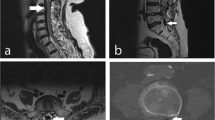
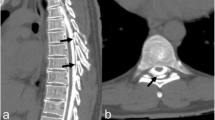
A 72-year-old woman, with no significant past medical history, presented with a 1 year history of thoracic back pain and a 2 week history of progressive lower extremity weakness. Neurological examination revealed MRC grade 3/5 power including and distal to the L2 myotome. She had reduced pin-prick sensation below L1 bilaterally. Reflexes were brisk in the lower limbs and both planters were upgoing. Neurological examination of the upper limbs was normal. Magnetic resonance imaging (Fig. 1) and a CT scan (Fig. 2) demonstrated a central calcified disc herniation at the level T9–T10 level causing spinal cord compression with T2 signal change in the spinal cord. The calcified disc prolapse occupied more than 60 % of the cross-sectional area of the spinal canal.
We performed transthoracic discectomy with continuous intraoperative neurophysiologic monitoring (SSEPs and MEPs). The patient was placed in a right lateral position. A left-sided minimally invasive lateral transthoracic transpleural approach between the 9th and 10th ribs was used. After the retractor system (MaXcess, NuVasive, Inc., San Diego, CA, USA) was introduced, the pleura was incised and dissected. The left 10th rib head was removed to allow visualisation of the posterolateral aspect of the disc and the vertebral body. Subsequently, we performed a wedge-shaped osteotomy of the vertebral body and cranial aspect of the T10 pedicle to allow exposure of the dura and calcified disc prolapse. Under the microscope we began careful dissection of the calcified disc. It became evident at this stage that the herniated disc prolapse was firmly adherent to the dura and had a transdural component, necessitating resection of the surrounding dura to allow safe and complete removal of the disc prolapse whilst protecting the spinal cord. The dural defect was sealed with three layers of dural substitute (TachoSil, Baxter), fibrin glue (Tisseel, Baxter) and a fourth layer of gelatine sponge (Spongistan, Johnson & Johnson). After the dural repair was complete, no CSF leakage was observed with valsalva manoeuver. The pleura was sutured to provide an additional layer of coverage. A chest drain with water seal chamber and without suction was placed prior to the closure of the thoracotomy wound.
Following surgery, the patient was kept sedated and ventilated on the intensive care unit for a period of 24 h. The ventilator was set on Bilevel positive airway pressure (BiPAP)—Assisted Spontaneous Breathing (ASB) with FiO2 of 0.4, Positive End-Expiratory Pressure (PEEP) set to 6 cmH2O and ASB peak of 16 cmH2O. Sedation was stopped and the endotracheal tube was removed on the second day after surgery. Examination of the chest and chest radiograph at this stage showed lung re-expansion and no evidence of pleural effusion.
Fluid output from the chest drain was moderate during the first 48 h following surgery, measuring a total of 250 ml. Over the subsequent 24 h only a further 20 ml was produced. The patient was stable and comfortable at this stage.
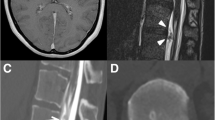
On the fourth postoperative day, however, the patient developed chest pain, dyspnoea and headache. The chest drain output increased to a total of 700 ml of clear fluid over the next 24 h and chest radiograph revealed bilateral pleural effusion (Fig. 3). The fluid drained from the chest drain tested positive for β2-transferrin (a sensitive marker for CSF). These findings led to the diagnosis of a subarachnoidal pleural fistula.
To manage the CSF fistula, we inserted a lumbar CSF drain and aimed to drain 10 ml/h. The patient was kept flat in bed. Despite these measures, the chest drain continued to drain large amounts of CSF and the patient’s respiratory function deteriorated, requiring increasing levels of inspired oxygen. Due to the cerebrospinal fluid depletion, the patient had worsening headache with nausea and dizziness. Noninvasive positive pressure ventilation (NPPV) with a face-mask was applied to counteract the inspiratory negative intrapleural pressure, which was promoting flow through the CSF fistula. In the following days, the symptoms resolved and the fluid output through the chest tube reduced. We were subsequently able to clamp the chest drain for a couple of days. The patient’s condition remained stable and the chest radiograph showed a substantial reduction in the pleural effusion allowing removal of chest drain and discontinuation of NPPV. The lumbar drain was removed after a two more days. The follow-up X-radiographs of the chest revealed no further pleural collection and the patient recovered well.
Discussion
Subarachnoid pleural fistula (SPF) is an abnormal communication between the subarachnoid and pleural space, which occurs in the presence of coexisting defects in the arachnoid and dura mater as well as the parietal pleura [1]. SPF can arise from blunt or penetrating trauma [2], as a complication of thoracic and spinal surgery [3], or spontaneously [4]. Pleural pressure is generally below atmospheric pressure (range between −2 and −8 cmH2O). During inspiration the intrapleural pressure becomes most negative. Given the positive pressure within the subarachnoid space CSF flows along the pressure gradient in the presence of a SPF and accumulates within the pleural space [5]. As 500 ml of CSF is produced per day in human adults there is a continuous flow of CSF preventing a spontaneous closure of the fistula. The presence of a chest tube with water seal chamber promotes flow through the fistula by removing CSF collecting in thoracic cavity [1].
The pleura is able to absorb CSF but its ability to do so can be overwhelmed, leading to a pleural collection. An example of this can be seen with ventriculopleural shunts, a long established treatment of hydrocephalus. The incidence of pleural effusion has been reported to range from 2–20 % in these cases [6]. Conversely small CSF leaks may go unnoticed. Fluid absorption by the pleural depends on pleural lymph flow in addition to hydraulic and osmotic forces. It is estimated that maximum lymph flow can potentially increase to 700 ml per day [7]. However, as non-clearance is determined by several factors, this rate of CSF clearance may not be possible.
In these cases clinical suspicion should be raised when symptoms of chest pain, dyspnoea, tachypnoea and cough appear. Chest radiographs usually confirm the presence of large pleural effusion. If the diagnosis remains uncertain additional investigation with ultrasound or computer tomography (CT) is recommended, as they are more sensitive at detecting small effusions [8].
Respiratory compromise due to a substantial pleural effusion may require thoracentesis or chest tube insertion; which allows lung re-expansion. To confirm SPF, pleural fluid should be tested of the presence of β2-transferrin [9], a biochemical marker that provides a high sensitivity (94–100 %) and specificity (98–100 %) for confirming presence of CSF [10]. Small CSF leaks can be difficult to identify, in which case CT myelography and radionuclide cisternography are indicated. Both have been reported as suitable and sensitive investigations to detect a SPF. Of these, CT myelography provides a better anatomical description whereas radionuclide cisternography has a higher sensitivity in detecting small SPF [1].
Drainage of large volumes of CSF through a SPF can lead to intracranial hypotension [11]; the symptoms of which are headache, nausea, vomiting, neck pain, changes in hearing and dizziness. In addition, there is a risk of subdural hematoma and cerebellar haemorrhage [12].
Disc herniation in the thoracic region comprises only 3 % of all disc herniation in the spine. Intradural disc herniations (IDH) are rare accounting for 0.26–0.3 % of all herniated discs [13]. Thoracic disc herniations are frequently calcified [3] which in itself raises the possibility of intradural encroachment. More than 60 % of calcified thoracic discs are found intraoperatively to have either an intradural component or are strongly adherent to (incorporated into) the dura [14]. Unfortunately there is no reliable diagnostic method to confirm intradural encroachment of a thoracic disc prior surgery. Magnetic resonance (MRI) with Gadolinium and CT can only give an indication as to whether this is likely; unless, as is very rarely encountered, an extradural CSF collection is visible [11], in which case one can be certain of a dural defect. It is vital that in all cases of calcified thoracic disc, one makes provisions for managing a dural defect and CSF leak in the pre-operative planning prior to embarking on surgery.
In the case described in our report we chose a transthoracic approach due to the central location of the disc herniation, its calcified nature and the patient’s symptoms of myelopathy. Several authors have reported on the advantages of an anterolateral transthoracic approach, particularly for centrally located calcified discs [15–19]. Other approaches have been reported, including costotransversectomy and posterior; however, we suggest that anterior approaches provide the best access to resect almost all thoracic disc herniations and also provide the best exposure for repair of a dural defect.
Cerebrospinal fluid leakage during transthoracic disc surgery has been reported in up to 15 % of the cases [3], and is either due to intradural disc herniation or iatrogenic causes. When identified intraoperatively, every effort should be made to repair the leak so as to prevent serious pulmonary and neurological complications in the postoperative period. The dural defect may be large and a watertight primary closure may not be possible. Alternative means of closure have been reported including multilayer techniques with the use of dural patches, fibrin glue or muscle patches. Some authors recommended the routine intraoperative placement of a lumbar drain whenever a dural defect is encountered. However, this is not necessary if a good closure with watertight seal is achieved during surgery. A lumbar drain can be subsequently inserted if a significant SPF is diagnosed post-operatively. If insertion is indicated we agree with the recommendation that the lumbar drain is kept in for several days after the chest drain is removed [20].
The use of prophylactic antibiotics is controversial, but as with other CSF leaks the body of evidence goes against prophylactic use to avoid infections with resistant organisms [1].
Positive-pressure ventilation (PPV) with a bilevel positive airway pressure (BiPAP) has been suggested as a beneficial intervention in managing SPF [21–23]. It is suggested that this counteracts the negative pleural pressure and promotes a spontaneous closure of the dura [1]. Kurata et al. treated two patients with SPF following anterior thoracic spine surgery and showed a successful closure of the CSF leakage after 14 days of NPPV in one patient and after 5 days in the other [23]. Yoshor et al. also report a case where use of NPPV was necessary for 5 days [21]. Our experience was similar, as 6 days of NPPV was necessary (in addition to lumbar drainage) for the CSF leak to close. Although only a handful of reports have been published, the most effective way of conservatively treating a SPF appears to be with nonivasive positive pressure ventilation (NPPV) plus lumbar CSF drainage.
If conservative management fails after 7–10 days, if severe symptoms persist or there is clinical deterioration, surgical treatment must be considered [1]. The target of revision surgery is the identification of the leakage and watertight closure.
References
Hentschel SJ, Rhines LD, Wong FC et al (2004) Subarachnoid-pleural fistula after resection of thoracic tumors. J Neurosurg 100 (4 Suppl Spine):332–336
Lloyd C, Sahn SA (2002) Subarachnoid pleural fistula due to penetrating trauma: case report and review of the literature. Chest 122(6):2252–2256
McCormick WE, Will SF, Benzel EC (2000) Surgery for thoracic disc disease. Complication avoidance: overview and management. Neurosurg Focus 9(4):e13
Kumar V, Bundela YS, Gupta V et al (2010) Spontaneous subarachnoid pleural fistula: a rare complication of lateral thoracic meningocele. Neurol India 58(3):466–467
Shamji MF, Sundaresan S, Da Silva V et al (2011) Subarachnoid-pleural fistula: applied anatomy of the thoracic spinal nerve root. ISRN Surg 2011:168959
Kupeli E, Yilmaz C, Akcay S (2010) Pleural effusion following ventriculopleural shunt: case reports and review of the literature. Ann Thorac Med 5(3):166–170
Miserocchi G (1997) Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 10(1):219–225
Moy MP, Levsky JM, Berko NS et al (2013) A new, simple method for estimating pleural effusion size on CT scans. Chest 143(4):1054–1059
Deseyne S, Vanhouteghem K, Hallaert G et al (2015) Subarachnoidal-pleural fistula (SAPF) as an unusual cause of persistent pleural effusion. Beta-trace protein as a marker for SAPF. Case report and review of the literature. Acta Clin Belg 70(1):53–57
Huggins JT, Sahn SA (2003) Duro-pleural fistula diagnosed by beta2-transferrin. Respiration 70(4):423–425
Winter SC, Maartens NF, Anslow P et al (2002) Spontaneous intracranial hypotension due to thoracic disc herniation. Case report. J Neurosurg 96(3 Suppl):343–345
Paldino M, Mogilner AY, Tenner MS (2003) Intracranial hypotension syndrome: a comprehensive review. Neurosurg Focus 15(6):ECP2
Epstein NE, Syrquin MS, Epstein JA et al (1990) Intradural disc herniations in the cervical, thoracic, and lumbar spine: report of three cases and review of the literature. J Spinal Disord 3(4):396–403
Gille O, Soderlund C, Razafimahandri HJ et al (2006) Analysis of hard thoracic herniated discs: review of 18 cases operated by thoracoscopy. Eur Spine J 15(5):537–542
Nacar OA, Ulu MO, Pekmezci M et al (2013) Surgical treatment of thoracic disc disease via minimally invasive lateral transthoracic trans/retropleural approach: analysis of 33 patients. Neurosurg Rev 36(3):455–465
Vollmer DG, Simmons NE (2000) Transthoracic approaches to thoracic disc herniations. Neurosurg Focus 9(4):e8
Ayhan S, Nelson C, Gok B et al (2010) Transthoracic surgical treatment for centrally located thoracic disc herniations presenting with myelopathy: a 5-year institutional experience. J Spinal Disord Tech 23(2):79–88
Quraishi NA, Khurana A, Tsegaye MM et al (2014) Calcified giant thoracic disc herniations: considerations and treatment strategies. Eur Spine J 23(Suppl 1):S76–S83
Russo A, Balamurali G, Nowicki R et al (2012) Anterior thoracic foraminotomy through mini-thoracotomy for the treatment of giant thoracic disc herniations. Eur Spine J 21(Suppl 2):S212–S220
Dickman CA, Rosenthal D, Regan JJ (1999) Reoperation for herniated thoracic discs. J Neurosurg 91(2 Suppl):157–162
Yoshor D, Gentry JB, Lemaire SA et al (2001) Subarachnoid-pleural fistula treated with noninvasive positive-pressure ventilation. Case report. J Neurosurg 94(2 Suppl):319–322
Valla FV (2007) Subarachnoid-pleural fistula in an infant treated with mechanical positive-pressure ventilation. Pediatr Crit Care Med 8(4):386–388
Kurata Y, Yoshimoto M, Takebayashi T et al (2010) Subarachnoid-pleural fistula treated with noninvasive positive pressure ventilation: a two-case report and literature review. Spine (Phila Pa 1976) 35 (18):E908–E911
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Patient consent statement
The patients’ next of kin have consented to the submission of the case report for submission to the journal.
Rights and permissions
About this article
Cite this article
Schlag, H.R., Muquit, S., Hristov, T.B. et al. Subarachnoidal pleural fistula after resection of intradural thoracic disc herniation and multimodal treatment with noninvasive positive pressure ventilation (NPPV). Eur Spine J 25, 155–159 (2016). https://doi.org/10.1007/s00586-015-4137-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-4137-1