Abstracts
Purpose
Evidence has shown that osteoporosis or intervertebral disc degeneration (IDD) led to cartilage endplate lesions (CEL), but their combined effects on the lesion remain unknown. This study developed an innovative rat model combined ovariectomy (OVX) and cervical muscle section (CMS), and aimed to evaluate the combined effects of osteoporosis and IDD on cartilage endplate lesions of cervical spine.
Methods
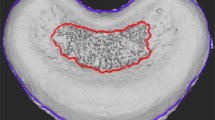
Fifty-two Sprague–Dawley female rats were assigned randomly into four groups as follows: the sham group (n = 10) underwent sham surgery; the OVX group (n = 14) was subjected to bilateral ovariectomy; the CMS group (n = 14) had posterior paraspinal muscles cut from C2 to C7; the CMS–OVX group (n = 14) underwent the OVX and CMS surgeries consecutively. Samples of C6–C7 segments were harvested at 12, 18 and 24 weeks post-surgery. Micro-CT analysis was performed to evaluate the CEL, intervertebral disc height (IDH) and structural indices. Histological analysis with Safranine O/fast green stain and histological score were used to observe the characteristics of the degenerative discs.
Results
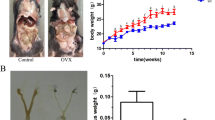
Ovariectomy surgery resulted in significant changes of most structural indices of the C6 body, such as decrease of percent bone volume and number of bone trabecula at 12 weeks, and greater changes at 18 and 24 weeks. The CEL following CMS surgery was seen on the ventral, while the CEL in the OVX and sham groups on the peripheral. The CEL was greatest in the CMS–OVX group and significantly greater than that in the CMS and OVX groups at 12 and 18 weeks (P < 0.05). The CMS surgery resulted in significant IDH decrease at 12, 18 and 24 weeks (P < 0.05), while the OVX surgery resulted in mild IDH decrease when compared with the sham group. The IDH in the CMS–OVX group was significantly lower than that in the CMS group at 24 weeks (P < 0.05). Histological evaluation suggested cartilage endplate abrasion at 12 weeks, and in situ calcification at 18 and 24 weeks in the CMS and CMS–OVX groups. Disc degenerative scores were higher following CMS or OVX surgery, and correlated with the CEL and IDH (P < 0.01), respectively.
Conclusions
The present study suggested that a combination of OVX and CMS led to more lesion of cartilage endplate than any one thereof, as well as more decrease of IDH. The lesion and IDH decrease were associated with the disc degeneration levels. The cartilage endplate was worn out at the early stage and calcified in situ later. The results indicate that osteoporosis may deteriorate the disc degeneration at specific time.






Similar content being viewed by others
References
Yoshimura N, Muraki S, Oka H, Mabuchi A et al (2009) Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab 27(5):620–628
Miyakoshi N, Itoi E, Murai H, Wakabayashi I, Ito H, Minato T (2003) Inverse relation between osteoporosis and spondylosis in postmenopausal women as evaluated by bone mineral density and semiquantitative scoring of spinal degeneration. Spine (Phila Pa 1976) 28:492–495
Nanjo Y, Morio Y, Nagashima H et al (2003) Correlation between bone mineral density and intervertebral disk degeneration in pre- and postmenopausal women. J Bone Miner Metab 21(1):22–27
Margulies JY, Payzer A, Nyska M et al (1996) The relationship between degenerative changes and osteoporosis in the lumbar spine. Clin Orthop Relat Res 324:145–152
Zhang Y, Xia J, Qiu Y et al (2012) Correlation between osteoporosis and degeneration of intervertebral discs in aging rats. J Huazhong Univ Sci Technolog Med Sci 32(2):210–215
Simpson EK, Parkinson IH, Manthey B et al (2001) Intervertebral disc disorganization is related to trabecular bone architecture in the lumbar spine. J Bone Miner Res 16(4):681–687
Wang YX, Griffith JF, Ma HT et al (2011) Relationship between gender, bone mineral density, and disc degeneration in the lumbar spine: a study in elderly subjects using an eight-level MRI-based disc degeneration grading system. Osteoporos Int 22(1):91–96
Gruber HE, Gordon B, Williams C et al (2003) Bone mineral density of lumbar vertebral end plates in the aging male sand rat spine. Spine 28(16):1766–1772
Wang T, Zhang L, Huang C et al (2004) Relationship between osteopenia and lumbar intervertebral disc degeneration in ovariectomized rats. Calcif Tissue Int 75(3):205–213
Luo Y, Zhang L, Wang WY et al (2013) Alendronate retards the progression of lumbar intervertebral disc degeneration in ovariectomized rats. Bone 55(2):439–448
Polikeit A, Nolte LP, Ferguson SJ (2004) Simulated influence of osteoporosis and disc degeneration on the load transfer in a lumbar functional spinal unit. J Biomech 37(7):1061–1069
Urban JPG, Smith S, Fairbank JCT (2004) Nutrition of the intervertebral disc. Spine 29(23):2700–2709
Gruber HE, Gordon B, Williams C et al (2007) Vertebral endplate and disc changes in the aging sand rat lumbar spine: cross-sectional analyses of a large male and female population. Spine 32(23):2529–2536
Roberts S, Menage J, Urban JPG (1989) Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine 14:166–174
Setton LA, Zhu W, Weidenbaum M et al (1993) Compressive properties of the cartilaginous end-plate of the baboon lumbar spine. J Orthop Res 11:228–239
Bernick S, Cailliet R (1982) Vertebral end-plate changes with aging of human vertebrae. Spine 7(2):97–102
Roberts S, Menage J, Eisenstein SM (1993) The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res 11:747–757
Gruber HE, Ashraf N, Kilburn J, Williams C, Norton HJ, Gordon BE, Hanley EJ (2005) Vertebral endplate architecture and vascularization: application of micro-computerized tomography, a vascular tracer, and immunocytochemistry in analyses of disc degeneration in the aging sand rat. Spine 30(23):2593–2600
Wang Y, Videman T, Battie MC (2012) ISSLS prize winner: lumbar vertebral endplate lesions: associations with disc degeneration and back pain history. Spine 37(17):1490–1496
Buser Z, Kuelling F, Liu J et al (2011) Biological and biomechanical effects of fibrin injection into porcine intervertebral discs. Spine (Phila Pa 1976) 36:E1201–E1209
Melrose J, Shu C, Young C et al (2012) Mechanical destabilisation induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration. Spine (Phila Pa 1976) 37(1):18–25
Liang QQ, Cui XJ, Xi ZJ, Bian Q, Hou W, Zhao YJ, Shi Q, Wang YJ (2011) Prolonged upright posture induces degenerative changes in intervertebral discs of rat cervical spine. Spine (Phila Pa 1976) 36:E14–E19
Wang YJ, Shi Q, Lu WW et al (2006) Cervical intervertebral disc degeneration induced by unbalanced dynamic and static forces: a novel in vivo rat model. Spine 31(14):1532–1538
Laing AC, Cox R, Tetzlaff W et al (2011) Effects of advanced age on the morphometry and degenerative state of the cervical spine in a rat model. Anat Rec 294:1326–1336
Han B, Zhu K, Li FC et al (2008) A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine 33(18):1925–1934
Moore RJ, Vernon-Roberts B, Fraser RD et al (1996) The origin and fate of herniated lumbar intervertebral disc tissue. Spine 21(18):2149–2155
Schmid G, Witteler A, Willburger R et al (2004) Lumbar disk herniation: correlation of histologic findings with marrow signal intensity changes in vertebral endplates at MR imaging. Radiology 231(2):352–358
Willburger RE, Ehiosun UK, Kuhnen C et al (2004) Clinical symptoms in lumbar disc herniations and their correlation to the histological composition of the extruded disc material. Spine 29(15):1655–1661
Rajasekaran S, Bajaj N, Tubaki V et al (2013) The anatomy of failure in lumbar disc herniation: an in vivo, multimodal, prospective study of 181 subjects. Spine 38(17):1491–1500
Stokes IA, Iatridis JC (2004) Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine 29(23):2724–2732
Laffosse JM, Accadbled F, Molinier F et al (2010) Correlations between effective permeability and marrow contact channels surface of vertebral endplates. J Orthop Res 28(9):1229–1234
Bian Q, Liang QQ, Wan C, Hou W et al (2011) Prolonged upright posture induces calcified hypertrophy in the cartilage end plate in rat lumbar spine. Spine 36(24):2011–2020
Chen B, Fellenberg J, Wang H et al (2005) Occurrence and regional distribution of apoptosis in scoliotic discs. Spine 30(5):519–524
Ariga K, Miyamoto S, Nakase T et al (2001) The relationship between apoptosis of endplate chondrocytes and aging and degeneration of the intervertebral disc. Spine 26(22):2414–2420
Ding Y, Jiang J, Zhou J et al (2013) Effects of imbalance of dynamic and static forces on the disc degeneration of cervical spine at different levels in rats: a micro-CT morphology and histology study. Chin J Spine Spinal Cord 7:638–643
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, Y., Jiang, J., Zhou, J. et al. The effects of osteoporosis and disc degeneration on vertebral cartilage endplate lesions in rats. Eur Spine J 23, 1848–1855 (2014). https://doi.org/10.1007/s00586-014-3324-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3324-9