Abstract
Altered dorsal root ganglion (DRG) function is associated with neuropathic pain following spinal nerve injury. However, compression of the cauda equina and dorsal rhizotomy proximal to the DRG do not induce significant pain, whereas in the spinal nerve and peripheral nerve, injury distal to the DRG does induce neuropathic pain. Caspase signaling induces apoptosis, and caspase inhibitors prevent pain-related behavior. The degree of DRG neuronal apoptosis is thought to play a role in pain behavior. We suggest that differences in pain behavior according to the injury sites within the DRG may be related to imbalances in apoptotic injuries. The aim of this study was to determine which compression injury was more painful and to compare behavior with expression of tumor necrosis factor (TNF)-alpha in DRG and apoptosis in the DRG following crush injury to the L5 nerve root or L5 spinal nerve. Sprague–Dawley rats received a crush injury to the L5 spinal nerve (distal to the DRG), crush injury to the L5 nerve root (proximal to the DRG), or no crush injury (sham). Mechanical allodynia was determined by the von Frey test. Expression of TNF-alpha was compared among three groups using immunoblot findings. Furthermore, we compared the percentage of neurons injured in the DRG using immunostaining for apoptotic cells and localization of activated caspase 3. Mechanical allodynia was observed in both crush injury groups. The duration of mechanical allodynia in the distal crush group was significantly longer than in the proximal crush group (P < 0.05). TNF-alpha expression was increased in DRG neurons following injury. DRG apoptosis in the distal crush group was significantly higher than in the proximal group at each time point (P < 0.05). This study suggests that spinal nerve crush injuries produce a greater degree of DRG apoptosis than do corresponding nerve root crush injuries, and that the former injuries are associated with longer lasting mechanical allodynia. Thus, differences in the time course of mechanical allodynia might be associated with an imbalance in DRG apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although lumbar radiculopathy leads to nociceptive pain, its mechanisms are not completely known. Animal models indicate that axonal degeneration following peripheral nerve injury causes neuropathic pain [15, 16], but demyelination alone does not [17, 24]. These effects are related to the production of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-alpha. TNF-alpha expression is upregulated in endoneurial macrophages and Schwann cells following axonal injury and results in neuropathic pain [38]. Injection of epidural, subcutaneous, or intraneural TNF-alpha produces allodynia [12, 39, 40]. Application of TNF-alpha to the nerve roots also produces neuropathologic changes and behavior deficits [8]. Further evidence that TNF-alpha is important in radiculopathy is the finding that inhibition of TNF-alpha by selective antibody therapy prevents changes in nerve pathology, such as thrombus formation and intraneural edema, reduction of nerve conduction velocity, and pain behavior [5, 18, 21, 22, 27, 28].
TNF-alpha is an important mediator of apoptosis of peripheral and central neurons because TNF-alpha receptor 1 (p55 receptor), which is associated with apoptosis and cytokine production, is upregulated in dorsal root ganglion (DRG) after peripheral nerve injury [23]. Nerve crush injury 2 mm distal to the DRG induces Wallerian degeneration and DRG apoptosis [1]. Likewise, nerve injury 2 mm proximal to the DRG induces apoptosis in the spinal cord and pain behavior, which anti-TNF-alpha therapy reduces [29]. We have reported previously that compression of the cauda equina (more than 2 mm proximal to the DRG) induces either demyelination or axonal degeneration depending on the degree of cauda equina compression, but does not induce neuropathic pain [30]. We also observed that the incidence of apoptosis was less in the DRG than in the dorsal horn after cauda equina compression [30]. Many studies have investigated a nerve root or spinal nerve injury in different kinds, degrees, or sites of injuries [2, 6, 9, 10, 31, 32]. A difference in pain behavior according to the injury site relates to findings in glial activation and DRG membrane excitability following nerve injury [2, 31]. The dorsal root rhizotomy, which is injured proximal to the DRG, has a shorter duration of hyperalgesia than peripheral nerve injury and does not result in pain-related behavior [31, 32]. These results suggest that the location of the injury relative to the DRG affects pain behavior and neuropathology. However, there is no report to compare the differences in pain behavior and pathological lesions according to injury sites with the same distance and degree of injury. The aim of this study was to compare pain behavior and apoptosis in the DRG following injury to nerve fibers immediately proximal or distal to the L5 DRG.
Materials and methods
A total of 129 adult female Sprague–Dawley rats (200–250 g) were used in this study. They were housed in plastic cages at room temperature in a 12:12-h light:dark cycle with free access to food and water. The animal experiment was carried out under the control of the Animal Care and Use Committee in accordance with Guidelines for the Animal Experiments of Fukushima Medical University and Japanese Government Law Concerning the Protection and Control of Animals.
Surgical procedure
Animals were anesthetized by intraperitoneal injection of 0.2 ml of sodium pentobarbital (50 mg/ml, Abbott Laboratories, Abbott Park, IL, USA). Animals were divided into three experimental groups: proximal crush group, distal crush group, and sham group. Animals in the crush group received a left L5 nerve root crush injury as follows: Rats were placed into a prone position, and an incision was made to the middle of the spine at the L4-L6 level. A deep dissection was made through the paraspinal muscles, which were then separated from the spinal processes at the L4-L6 level to reveal the L5 lamina by microscopy. The L6 transverse process was exposed for identification of the L5 nerve. The left L5 DRG was exposed after left L5/L6 facetectomy and L5 hemilaminectomy. The injury was created by crushing the nerve root 2 mm proximal to the DRG (proximal crush group) or the spinal nerve 2 mm distal to the DRG (distal crush group) with fine forceps one time for 3 s [29, 36]. Wounds were closed with 4.0 nylon (fascia) and surgical staples (skin), and the rats were allowed to recover in their normal environment. The animals in the sham group underwent the same surgical procedure expect for the left L5 nerve crush injury.
Behavioral testing for mechanical allodynia
Thirty animals (10 in each group) underwent behavior testing for the three experimental groups. Sensitivity to non-noxious mechanical stimuli was tested by determining the hind paw withdrawal response to von Frey hair stimulation of the plantar surface of the foot pad. Rats were acclimated to being placed on a suspended 6-mm wire grid and to having the plantar surface of their footpads stimulated with von Frey filaments. Nine filaments, calibrated between 1 and 29 g (1.20, 1.48, 2.04, 3.63, 5.50, 8.51, 11.75, 15.14, and 28.84 g) force were sequentially applied to the paw surface just until the filament bent, for a total of two applications approximately 2–3 s apart and varied in location so as to avoid sensitization. If the rat did not withdraw its foot after either of the two applications using a given filament, the next stiffer filament was tested in the same manner. When the rat did withdraw its foot, the measurement was verified by ensuring that there was an absence of response at the next lower filament. The gram force of the filament causing the positive response was recorded after the first reaction. After 5 min the same procedure was performed again to verify the initial response [29, 36].
Baseline testing was done before the start of the experiment to acclimate the animals to the testing procedure and to verify that the animals had normal responses. Crush and sham animals were tested on 2, 7, 14, and 28 days after surgery.
Immunoblot for TNF
Immunoblot findings were analyzed using 39 rats that were not used for behavior testing. Animals were divided into four groups: proximal crush group, distal crush group, sham group (n = 12 in each group), and naïve animals (n = 3). Animals underwent surgeries according to the above-mentioned surgical procedure except for naïve animals. Animals were decapitated rapidly under anesthesia, and the corresponding DRG were removed and frozen by liquid nitrogen on postoperative day 2, 7, 14, and 28. Separate animals were used for each time point (n = 3 at each time point in each group). Naïve animals were used as a control. Samples were homogenized in a lysis buffer consisting of 20 mM Tris–HCl (pH 7.5), 150 mM Nacl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM b-glycerophosphate, 1 mM Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml trypsin inhibitor, and 100 μM phenylmethylsulfonyl fluoride, and reduced in an SDS sample buffer consisting of 62.5 mM Tris–HCL (pH 6.8), 2% SDS, 10% glycerol, 50 mM DTT, and 0.01% bromophenol blue. Protein samples were separated on SDS-PAGE and transferred to polyvinylidene difluoride filter membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk and incubated overnight at 4°C with a primary antibody. Primary antibodies used included mouse anti-rat TNF-alpha monoclonal antibody (1:200) (R&D Systems, Minneapolis, MN, USA) and mouse anti-b-actin monoclonal antibody (1:5,000; Sigma, St. Louis, MO, USA). Next, those membranes were incubated for 1 h at room temperature with HRP-conjugated secondary antibody (1:10,000) (Bio-Rad, Hercules, CA, USA). Recombinant rat TNF-alpha (R&D Systems), 0.6 ng/lane, was used as a positive control and 20% bovine serum albumin was used as a negative control. Positive bands were visualized using an enhanced chemiluminescence system (Amersham Biosciences, Piscataway, NJ, USA). The positive bands of immunoblots were analyzed by rates of internal control b-actin using a computer-assisted imaging analysis system (ImageJ version 1.13u, National Institutes of Mental Health, Bethesda, MD, USA) three times for each animal at each time point. Three measurements in each animal were used for the statistical analysis. Thus the mean and standard deviation of nine measurements (three measurements for each of three animals) in each group at each time point was used. Differences between individual membranes were standardized relative to the positive control.
Histology
Histological findings were analyzed using 60 rats that were not used for behavior testing. Animals in the distal and proximal crush groups and sham group (n = 20 in each group) were compared using fluorescent staining and quantification of apoptosis. Animals were anesthetized with diethyl ether (Wako Pure Chemical Industries, Osaka, Japan) and 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) was perfused through the ascending aorta at postoperative days 2, 7, 14, and 28 (n = 5 in each group at each time point). Separate animals were used for each time point. The left L5 DRG was removed and post-fixed briefly in 4% paraformaldehyde and subsequently embedded in paraffin. Two sections (6 μm each) from each DRG were cut and placed on slides.
Fluorescent staining and microscopy
Double-staining with cleaved caspase 3 and NeuN or GFAP antibodies was performed as follows. DRG samples were embedded in paraffin, sectioned (6 μm), and placed on slides. Sections were deparaffinized and rehydrated in a graded ethanol series. After blocking with 2% gelatin/PBS, dual-label immunofluorescence was performed by incubating paraffin sections with rabbit anti-cleaved caspase 3 antibody (1:200, Cell Signaling Technology Inc., Danvers, MA, USA), which was applied overnight at 4°C, followed by incubation with Alexa 555 (1:200) (red) fluorescent antibody (1:400; Molecular Probes Inc., Eugene, OR, USA) for 1 h at room temperature. Next, 5% normal goat serum was applied for 30 min. Subsequently, incubation with antibody to mouse anti- NeuN (1:200; Chemicon International, Temecula, CA, USA) or GFAP (1:10; Progen, Heidelberg, Germany) was performed overnight at 4°C. Sections were rinsed in PBS and incubated for 1 h at room temperature with Alexa Fluor 488 (green; 1:200; Molecular Probes). Fluorescent staining was analyzed using an Olympus Optical BX50 fluorescent microscope with imaging software, Axio Vision ver. 4.4 (Carl Zeiss, Gettingen, Germany).
In situ Oligo labeling (ISOL) for apoptosis
DRG samples embedded in paraffin were cut in transverse sections (10 μm) and placed on slides. Two sections for each animal were chosen, and DRG sections were assayed for apoptosis using an ApoTag, in situ Oligo Ligation Kit (Chemicon), a technique based on the biochemical specificity of the enzyme T4 ligase, which specifically distinguishes apoptotic neurons from necrotic neurons. Slides were counterstained with methyl green. A positive control slide provided by the manufacturer was also stained.
Quantification of apoptotic cells
The apoptotic cell bodies of DRG were counted at 400× magnification for each section. The number of brown-stained apoptotic neurons was divided by the total number of green and brown stained neurons within the field and multiplied by 100.
Statistical analysis
Behavioral data were analyzed by analysis of variance with both non-parametric and multiple comparisons by Bonferroni correction test among the three treatment groups. The expressions of immunoblotting for TNF at each time point were analyzed by the Tukey-Kramer test. Comparisons of the number of apoptosis-positive cells were performed by the Bonferroni correction test. Critical values that corresponded to a P value < 0.05 were considered significant. All data are reported as mean ± SD.
Results
Behavior tests
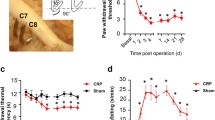
The mechanical withdrawal threshold on the ipsilateral hind paw for mechanical allodynia using the von Frey test is shown in Fig. 1. In the distal crush group (n = 10), the mechanical withdrawal threshold decreased for 28 days, whereas, in the proximal crush group (n = 10), the threshold decreased for 14 days and recovered after 14 days. In the sham group (n = 10), the threshold did not decrease for 28 days compared with baseline. The decrease in mechanical threshold on the ipsilateral hind paw in both crush groups was significantly greater than the sham group on day 14 (P < 0.001). However, the threshold in the distal crush group was significantly lower than in the sham group (P < 0.0033), and there was no significant difference in the threshold between the proximal crush and sham groups on day 28 (P = 0.3408). For animals in which the threshold was less than 5 g, more than 60% of ipsilateral hind paws exhibited mechanical allodynia at 28 days post surgery in the distal groups, whereas less than 30% of the paws exhibited mechanical allodynia after 14 days in the proximal group. The duration of mechanical allodynia in the distal crush group was significantly longer than in the proximal group (P < 0.05).
Behavior testing (n = 10 each group). This graph shows mechanical withdrawal threshold for 28 days of ipsilateral (left) hind paws in each group. Mechanical threshold decreased on the ipsilateral hind paw in both crush groups compared with the sham group at day 14 (P < 0.05). The threshold was significantly decreased in the distal crush group, but not in the proximal group compared with the sham group at day 28 (P = 0.0033, P = 0.3408, respectively). The duration of mechanical allodynia in the distal crush group was significantly longer than in the proximal crush group (P < 0.05)
Immunoblot of TNF-alpha in the DRG
TNF-alpha was observed in homogenized DRGs of the sham, proximal, and the distal crush groups for 28 days (n = 12 in each group) (Fig. 2). The level of TNF-alpha in the sham group was not significantly different from the level in the naïve group. At day 2, the levels of TNF-alpha in both crush groups were higher than in the sham group (P < 0.05), whereas there were no differences in levels of TNF-alpha between the distal and proximal crush groups. The level of TNF-alpha in the distal crush group was higher than the levels in both the TNF-alpha proximal crush and sham group at days 7, 14, and 28 (P < 0.05 at each time point). In the proximal crush group, the level of TNF-alpha was significantly higher than the sham group from 2 to 14 days (P < 0.05 at each time point) but not at day 28.
Immunoblot of TNF in DRG (n = 3 with three measurements per animal at each time point). The levels of TNF in the distal group were higher than in the proximal group at days 7, 14, and 28 (p < 0.05). The level of TNF in the sham group was significantly lower than in both crush groups at day 14, but there was no significant difference in TNF level between the sham and proximal groups at day 28. Sh sham, dis distal, pro proximal
Co-localization of activated caspase 3 in DRG
In both crush groups, activated caspase 3 (green) was observed (Fig. 3b, e), and was co-localized with NeuN (Fig. 3c) and GFAP (Fig. 3f). These results suggested that expression of activated caspase 3, which leads to apoptosis, was located in both DRG cell bodies and satellite cells. There was no difference in localization of caspase 3 between groups.
Fluorescent of cleaved caspase 3 in DRG. Activated caspase 3 (green) is an indicator of apoptosis and was observed by fluorescent staining in the crush groups (b, c). Activated caspase 3 was co-located on DRG neurons (c) and satellite cells (f). NeuN (red) is a marker of neurons (a). GFAP (red) is a marker of activated satellite cells (d)
Quantification of apoptosis cells in DRG
Apoptotic cells were observed in the ipsilateral DRGs of both crush and sham groups (n = 20 in each group, n = 5 at each time point) (Fig. 4). The percentage of apoptotic cells in the distal crush group was significantly higher than in the proximal crush group at each time point (P < 0.05). The percentage of apoptotic cells in the sham group was significantly lower than in the distal and proximal groups for day 14 (P < 0.05); however, there was no significant difference between the proximal and sham groups at day 28.
The percentage of apoptotic cells in ipsilateral DRG. Apoptosis neurons by ISOL-positive cells were counted and expressed as a percentage of total neurons per section. Ten samples at each time point were analyzed from three groups. The percentage of apoptotic cells in the distal group was higher than in the proximal group at each time point (P < 0.05). The percentage of apoptotic cells in the sham group was significantly lower than in both crush groups at day 14 (P < 0.05). However, there were no significant differences between the sham and proximal crush groups
Discussion
Lumbar radiculopathy associated with disc prolapse and lateral recess stenosis is the most common cause of nociceptive pain and generally means that tissue proximal to the DRG is injured. On the other hand, many cases of central canal stenosis induce various types of dysfunction, such as intermittent claudication without leg pain associated with cauda equina syndrome. The relationship of DRG neuronal apoptosis has been studied with respect to the distance between the DRG and a distal lesion, where it is known that lesions close to the DRG produce more apoptosis [1, 29]. Proximal to the DRG, injuries to the long fibers of the cauda equina, where the location of nerve lesions is more distant to the DRG, do not cause a significant reduction of mechanical pain threshold [9, 10, 30]. These results are similar to those of patients with central canal stenosis. Many studies have investigated a nerve root or spinal nerve injury in different kinds, degrees, and/or sites of injuries [2, 6, 9, 10, 31, 32]. The dorsal root rhizotomy, which is injured proximal to the DRG, has a shorter duration of hyperalgesia than peripheral nerve injuries and does not result in pain-related behavior [31, 32]. These results suggest that changes in behavior, histological, and electrophysiological findings in the nerve root proximal to a DRG injury are different compared with a peripheral nerve injury. However, there is no report to compare the differences in pain behavior and pathological lesions according to the injury sites with the same distance and degree of injury. To better understand these phenomena, we investigated apoptosis of the DRG after nerve injury, using the same crush technique at an equal distance (2 mm) proximal and distal to the DRG. The withdrawal threshold to von Frey stimulation of the ipsilateral hind paw in both groups decreased after nerve injury. There was no difference in threshold changes in the two lesions. However, the number of animals that exhibited pain with a distal crush injury was higher than the number that experienced pain with a proximal crush injury, and the duration of pain behavior following distal nerve injury continued significantly longer than that following proximal injury. The threshold showed high variability in the proximal group at Day 28, and the sham group at each time point. This result may have been due to von Frey filaments, as the width of filament calibrations was not consistent. In particular, the width between the two thicker filaments was 13.7 g, whereas it was only 0.28 g between the two thinner filaments. This may explain why the standard deviations were wider and the thresholds tended to show higher variability in the sham and proximal groups at day 28 compared with the distal and proximal crush group at day 14.
In this study, pain behavior, expression and amount of TNF-alpha in DRG, and the percentage of DRG neurons undergoing apoptosis, were different between animals receiving DRG distal and proximal crush lesions. Results were significantly increased in the distal crush group compared with the proximal crush group. Because the DRG was not injured directly, the resulting apoptosis was secondary to the nerve injuries. The mechanism of this differential response is unclear. One important factor may relate to cytokine signaling mechanisms of apoptosis secondary to retrograde axonal transport of proinflammatory cytokines [33, 34] and activation of neuronal apoptosis pathways [3, 13]. In this study, the amount of TNF-alpha in the DRG was related to the rate of apoptosis in DRG neurons from day 7 to day 28. Apparently, injuries to the nerve fibers during both DRG proximal and distal crush injured produce similar TNF-alpha activation, while injuries immediately distal to the DRG produce an additional significant increase in DRG neuronal apoptosis. Activated caspase 3 was expressed in both DRG neurons and satellite cells. Caspase 3 is one of the key indicators of apoptosis, as it is responsible for the proteolytic cleavage of many key proteins. A previous paper reported that caspase inhibitors prevent pain-related behavior; therefore, caspase signaling pathways can contribute to pain [13, 35]. Our results suggest that the number of apoptotic neurons in the DRG is directly related to pain behavior. We did not investigate the ratio of apoptosis in neurons and satellite cells respectively. However, caspase 3 was expressed in GFAP immunoreactive satellite cells in the present study. The expression of GFAP immunoreactive satellite cells has been shown to increase after peripheral nerve injury and spinal nerve injury [7, 23]. TNF-alpha, TNF-alpha receptor, nerve growth factor, and neurotrophin-3 are expressed in satellite cells [7, 23, 41]. In addition, TNF-alpha co-localized with GFAP-positive satellite cells was observed in the non-injury side DRG, which is related to the reduction of pain-related behavior [7]. Therefore, the apoptosis in activated satellite cells may be associated with inducing pain.
Because apoptotic cells in the proximal crush group did not increase at day 2 compared with the distal crush group, even though the TNF-alpha increased in both groups at this time point, one limitation of this study was that the pain threshold reduced at day 2 in both groups and we did not perform experiments before day 2. Differences in TNF-alpha quantity in the early phase, before 2 days, may need to be investigated to verify the reduction of the pain threshold. Furthermore, the discrepancy of pain behavior at day 2, the amount of TNF-alpha, and the delay in undergoing apoptosis might be caused by other factors such as blood flow supply to the DRG [11, 19, 20], nutritional pathway from cerebrospinal fluid (CSF) to nerve root [25], influences of the sympathetic nerve [4, 26], and spinal cord reaction from nerve injury. In particular, in terms of blood flow, apoptosis associated with activated caspase 3 is induced by ischemic changes [19]. Reduction of blood flow is induced after compression of nerve root [11]. In addition, blood flow supply in the nerve root proximal to the DRG is greater compared with blood flow distal to the DRG [20]. This result suggests that the blood flow supply in the distal crush injury is less than in the proximal crush injury and may be associated with differences in apoptosis ratio between both crush injuries in this study. In addition, if we consider CSF as another factor, it is known that nerve root is surrounded by CSF but not in the spinal or peripheral nerves. It reported that the nutritional supply for the spinal nerve root from CSF in 58% and intramural blood vessels in 35%, whereas that for the peripheral nerves from intramural blood vessels in 95% [25]. Therefore, the nutritional supply from CSF in the proximal crush injury may influence nerve damage or recovery compared with the distal crush injury. Another potential factor affecting our findings might be sympathetic sprouting in the DRG. It is reported that sympathetic sprouting in the DRG after nerve injuries affects DRG function. We previously reported that sympathectomy reduced both mechanical allodynia and DRG apoptosis in a proximal crush injury [36]. However, we did not compare pain-related behavior and apoptosis in the proximal and distal crush injuries with sympathectomy. According to a previous report, axonal transport of TNF-alpha after peripheral nerve injury is increased compared with normal animals [33, 34]. Furthermore, some reports show expression of pain-related substances, changes in morphological expression of substances such as P2X and Ephrin, and glial reactions after different types of nerve damage [7, 14, 37]. Expression of apoptosis in the spinal cord has also been found and is associated with pain- related behavior following spinal nerve or nerve root injuries [7, 29]. Another limitation of our study is that we did not compare the ratio of apoptosis in the spinal cord between the proximal and distal nerve injuries. Future studies must investigate these potential differences.
For the above-mentioned reasons, additional experimental studies are needed to investigate the mechanisms of pain behavior in both nerve root and spinal nerve injuries.
Conclusion
Nerve injuries both proximal and distal to the DRG induce mechanical allodynia. The duration of pain following distal crush injury is longer than that following proximal crush injury. The level of TNF-alpha expressed in DRG after distal nerve crush injury is higher than after proximal crush injury. Expression of DRG apoptosis in the distal crush injury was higher than in the proximal crush injury. These findings help explain the clinical observation that nerve root crush injuries proximal to the DRG, such as may occur in the cauda equina, are less painful than spinal nerve crush injuries. The mechanism for these results may relate to DRG TNF-alpha expression and apoptosis.
References
Campana WM, Myers RR (2003) Exogenous erythropoietin protects against dorsal root ganglion apoptosis and pain following peripheral nerve injury. Eur J Neurosci 18:1497–1506. doi:10.1046/j.1460-9568.2003.02875.x
Colburn RW, Richman AJ, DeLeo JA (1999) The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol 157:289–304. doi:10.1006/exnr.1999.7065
Chen M, Wang J (2002) Initiator caspases in apoptosis signaling pathways. Apoptosis 7:313–319. doi:10.1023/A:1016167228059
Chung K, Chung JM (2001) Sympathetic sprouting in the dorsal root ganglion after spinal nerve ligation: evidence of regenerative collateral sprouting. Brain Res 895:204–212. doi:10.1016/S0006-8993(01)02092-3
Goupille P, Mulleman D, Paintaud G, Watier H, Valat JP (2007) Can sciatica induced by disc herniation be treated with tumor necrosis factor alpha blockade? Arthritis Rheum 56:3887–3895. doi:10.1002/art.23051
Howard RF, Walker SM, Mota PM, Fitzgerald M (2005) The ontogeny of neuropathis pain: Postnatal onset of mechanical allodynia in rat sprea nerve injury (SNI) and chronic constriction injury (CCI) models. Pain 115:382–389. doi:10.1016/j.pain.2005.03.016
Hatashita S, Sekiguchi M, Kobayashi H, Konno S, Kikuchi S (2008) Contralateral neuropathic pain and neuropathology in dorsal root gandlion and spinal cord following hemilateral nerve injury in rats. Spine 33:1344–1351. doi:10.1097/BRS.0b013e3181733188
Igarashi T, Kikuchi S, Shubayev V, Myers RR (2000) 2000 Volvo Award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine 25:2975–2980. doi:10.1097/00007632-200012010-00003
Ito T, Ohtori S, Inoue G, Koshi T, Doya H, Ozawa T, Saito T, Moriya H, Takahashi K (2007) Glial phosphorylated p38 MAP kinase mediated pain in a rat model of lumbar disc herniation and induces motor dysfunction in a rat model of lumbar spinal canal stenosis. Spine 32:159–167. doi:10.1097/01.brs.0000251437.10545.e9
Ito T, Ohtori S, Hata K, Inoue G, Moriya H, Takahashi K, Yamashita T (2007) Rho kinase inhibitor improves motor dysfunction and hypoalgesia in a rat model of lumbar spinal canal stenosis. Spine 32:2070–2075. doi:10.1097/BRS.0b013e318145a502
Igarashi T, Yabuki S, Kikuchi S, Myers RR (2005) Effect of acute nerve root compression on endoneurial fluid pressure and blood flow in rat dorsal root ganglion. J Orthop Res 23:420–424. doi:10.1016/j.orthres.2004.08.026
Junger H, Sorkin LS (2000) Nociceptive and inflammatory effects of subcutaneous TNF alpha. Pain 85:145–151. doi:10.1016/S0304-3959(99)00262-6
Joseph EK, Levine JD (2004) Caspase signaling in neuropathic and inflammatory pain in the rat. Eur J Neurosci 20:2896–2902. doi:10.1111/j.1460-9568.2004.03750.x
Kobayashi H, Kitamura T, Sekiguchi M, Homma MK, Kabuuama Y, Konno S, Kikuchi S, Homma Y (2007) Involvement of EphB1 receptor/Ephrin B2 ligand in neuropathic pain. Spine 32:1592–1598. doi:10.1097/BRS.0b013e318074d46a
Myers RR, Heckman HM, Powell HC (1996) Axonal viability and the persistence of thermal hyperalgesia after partial freeze lesions of nerve. J Neurol Sci 139:28–38. doi:10.1016/S0022-510X(96)00038-X
Myers RR, Yamamoto T, Yaksh TL, Powell HC (1993) The role of focal nerve ischemia and Wallerian degeneration in peripheral nerve injury producing hyperesthesia. Anesthesiology 78:308–336. doi:10.1097/00000542-199302000-00015
Myers RR, Shubayev V, Campana WM (2001) Neuropathology of painful neuropathies. Pain Peripher Nerve Dis 13:8–30. doi:10.1159/000061872
Murata Y, Onda A, Rydevik B, Takahashi K, Olmarker K (2004) Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced histologic changes in the dorsal root ganglion. Spine 29:2477–2484. doi:10.1097/01.brs.0000144406.17512.ea
Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA (1998) Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci 18:3659–3668
Naito M, Owen JH, Bridwell K, Oakley D (1990) Blood flow direction in the lumbar nerve root. Spine 15:966–968. doi:10.1097/00007632-199009000-00023
Olmarker K, Nutu M, Storkson R (2003) Changes in spontaneous behavior in rats exposed to experimental disc herniation were blocked by selective TNF-alpha inhibition. Spine 28:1635–1642. doi:10.1097/00007632-200308010-00002
Olmarker K, Rydevik B (2001) Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve condition velocity. Possible implications for future pharmacologic treatment strategies of sciatica. Spine 26:863–869. doi:10.1097/00007632-200104150-00007
Ohtori S, Takahachi K, Moriya H, Myers RR (2004) TNF-alpha and TNF- alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury. Spine 29:1082–1088. doi:10.1097/00007632-200405150-00006
Powell HC, Myers RR (1986) Pathology of experimental nerve compression. Lab Invest 55:91–100
Rydevik B, Holm S, Brown MD, Lundborg G (1990) Diffusion from the cerebrospinal fluid as a nutritional pathway for spinal nerve roots. Acta Physiol Scand 138:247–248. doi:10.1111/j.1748-1716.1990.tb08843.x
Ramer MS, Bisby MA (1997) Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain 70:237–244. doi:10.1016/S0304-3959(97)03331-9
Sasaki N, Kikuchi S, Konno S, Sekiguchi M, Watanabe K (2007) Anti-TNF-alpha antibody reduces pain-behavioral changes induced by epidural application of nucleus pulposus in a rat model depending on the timing of administration. Spine 32:413–416. doi:10.1097/01.brs.0000255097.18246.bc
Schäfers M, Svensson C, Sommer C, Sorkin LS (2003) Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci 23:2517–2521
Sekiguchi Y, Kikuchi S, Myers RR, Campana WM (2003) ISSLS prize Winner: Erythropoietin inhibits spinal neuronal apoptosis and pain following nerve root crush. Spine 28:2577–2584. doi:10.1097/01.BRS.0000096674.12519.12
Sekiguchi M, Kikuchi S, Myers RR (2004) Experimental spinal stenosis relationship between degree of cauda equina compression, neuropathology, and pain. Spine 29:1105–1111. doi:10.1097/00007632-200405150-00011
Song XJ, Vizcarra C, Xu DS, Rupert RL, Wong ZN (2003) Hyperalgesia and neural excitability flowing injuries to central and peripheral branches of axons and somata of dorsal root ganglion neurons. J Neurophysiol 89:2185–2193. doi:10.1152/jn.00802.2002
Sheen K, Chung JM (1993) Signs of neuropathic pain depend on signals from injured nerve fibers in a rat model. Brain Res 30:62–68. doi:10.1016/0006-8993(93)91217-G
Shubayev V, Myers RR (2001) Axonal transport of TNF-alpha in painful neuropathy: distribution of ligand tracer and TNF receptors. J Neuroimmunol 114:48–56. doi:10.1016/S0165-5728(00)00453-7
Schäfers M, Geis C, Brors D, Yaksh TL, Sommer C (2002) Anterograde transport of tumor necrosis factor-alpha in the intact and injured rat sciatic nerve. J Neurosci 22:536–545
Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ (2005) Blocking caspase activity prevents transsynpatic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Nuerosci 25:7317–7323. doi:10.1523/JNEUROSCI.1526-05.2005
Sekiguchi M, Kobayashi H, Sekiguchi Y, Konno S, Kikuchi S (2008) Sympathectomy reduces mechanical allodynia, tumor necrosis factor-alpha expression, and dorsal root ganglion apoptosis following nerve root crush injury. Spine 33:1163–1169. doi:10.1097/BRS.0b013e31817144fc
Tsuda M, Toyomitsu E, Komatsu T, Masuda T, Kunifusa E, Tada KN, Koizumi S, Yamamoto K, Ando J, Inoue K (2008) Fibronection/integrin system is involved in P2X4 receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia 56:579–585. doi:10.1002/glia.20641
Wagner R, Myers RR (1996) Schwann cells produce tumor necrosis factor alpha: expression in injured and no-injured nerves. Neuroscience 73:625–629. doi:10.1016/0306-4522(96)00127-3
Wagner R, Myers RR (1996) Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport 7:2897–2901. doi:10.1097/00001756-199611250-00018
Zelenka M, Schäfers M, Sommer C (2005) Intraneural injection of interleukin-1 beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain 116:257–263. doi:10.1016/j.pain.2005.04.018
Zhou XF, Chie E, Xue Q, Zhong JH, McLachlan EM, Rush RA, Xian CJ (1999) Satellite-cell-derived nerve growth factor and neurotrophin-3 are involved in noradrenergic sprouting in the dorsal root ganglia following peripheral nerve injury in the rat. Eur J Neurosci 11:1711–1722. doi:10.1046/j.1460-9568.1999.00589.x
Acknowledgment
This study was supported by grant from the Fukushima Society for the Promotion of Medicine.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Sekiguchi, M., Sekiguchi, Y., Konno, Si. et al. Comparison of neuropathic pain and neuronal apoptosis following nerve root or spinal nerve compression. Eur Spine J 18, 1978–1985 (2009). https://doi.org/10.1007/s00586-009-1064-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-009-1064-z