Abstract
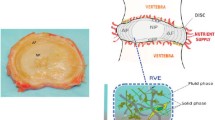
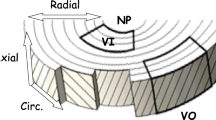
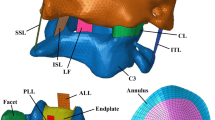
Intervertebral discs have a primarily mechanical role in transmitting loads through the spine. The disc is subjected to a combination of elastic, viscous and osmotic forces; previous 3D models of the disc have typically neglected osmotic forces. The fibril-reinforced poroviscoelastic swelling model, which our group has recently developed, is used to compute the interplay of osmotic, viscous and elastic forces in an intervertebral disc under axial compressive load. The unloaded 3D finite element mesh equilibrates in a physiological solution, and exhibits an intradiscal pressure of about 0.2 MPa. Before and after axial loading the numerically simulated hydrostatic pressure compares well with the experimental ranges measured. Loading the disc decreased the height of the disc and results in an outward bulging of the outer annulus. Fiber stresses were highest on the most outward bulging on the posterior-lateral side. The osmotic forces resulted in tensile hoop stresses, which were higher than typical values in a non-osmotic disc. The computed axial stress profiles reproduced the main features of the stress profiles, in particular the characteristic posterior and anterior stress which were observed experimentally.







Similar content being viewed by others
References
Op De Beeck R, Hermans D (2000) Research on work-related low back disorders. Report of European Agency for Safety and Health at work
Houben GB, Drost MR, Huyghe JM, Janssen JD, Huson A (1997) Nonhomogeneous permeability of canine anulus fibrosus. Spine 22:7–16
Urban JP, Maroudas A, Bayliss MT, Dillon J (1979) Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology 16:447–464
Urban JP, Roberts S (2003) Degeneration of the intervertebral disc. Arthritis Res Ther 5:120–130
Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE (1999) New in vivo measurements of pressures in the intervertebral disc in daily life. Spine 24:755–762
Cheung JT, Zhang M, Chow DH (2003) Biomechanical responses of the intervertebral joints to static and vibrational loading: a finite element study. Clin Biomech (Bristol, Avon) 18:790–799
Wu JS, Chen JH (1996) Clarification of the mechanical behaviour of spinal motion segments through a three-dimensional poroelastic mixed finite element model. Med Eng Phys 18:215–224
Elliott DM, Setton LA (2000) A linear material model for fiber-induced anisotropy of the anulus fibrosus. J Biomech Eng 122:173–179
Wagner DR, Lotz JC (2004) Theoretical model and experimental results for the nonlinear elastic behavior of human annulus fibrosus. J Orthop Res 22:901–909
Wang JL, Parnianpour M, Shirazi-Adl A, Engin AE (2000) Viscoelastic finite-element analysis of a lumbar motion segment in combined compression and sagittal flexion. Effect of loading rate. Spine 25:310–318
Nachemson A., Morris J (1963) Lumbar discometry. Lumbar intradiscal pressure measurements in vivo. Lancet 1:1140–1142
Ishihara H, McNally DS, Urban JP, Hall AC (1996) Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol 80:839–846
Neidlinger-Wilke C, Wuertz K, Claes L and Urban JP (2005) Osmolarity influences disc cell gene expressions in response to mechanical stimulation and at unloaded culture conditions. Eur Spine J 14(Supple 1):S29–S82 (Conference Proceeding)
Wognum S, Huyghe JM, Baaijens FPT (2006) Influence of osmotic pressure changes on the opening of existing cracks in two intervertebral disc models. Spine (accepted)
Lanir Y (1987) Biorheology and fluid flux in swelling tissues, II. Analysis of unconfined compressive response of transversely isotropic cartilage disc. Biorheology 24:189–205
Lanir Y (1987) Biorheology and fluid flux in swelling tissues. I. Bicomponent theory for small deformations, including concentration effects. Biorheology 24:173–187
Lai WM, Hou JS, Mow VC (1991) A triphasic theory for the swelling and deformation behaviors of articular-cartilage. J Biomech Eng Trans Asme 113:245–258
Huyghe JM, Janssen JD (1997) Quadriphasic mechanics of swelling incompressible porous media. Int J Eng Sci 35:793–802
Frijns AJH, Huyghe JM, Janssen JD (1997) A validation of the quadriphasic mixture theory for intervertebral disc tissue. Int J Eng Sci 35:1419–1429
van Loon R, Huyghe JM, Wijlaars MW, Baaijens FPT (2003) 3D FE implementation of an incompressible quadriphasic mixture model. Int J Numer Methods Eng 57:1243–1258
Wilson W, van Donkelaar CC, Huyghe JM (2005) A comparison between mechano-electrochemical and biphasic swelling theories for soft hydrated tissues. J Biomech Eng 127:158–165
Iatridis JC, Laible JP, Krag MH (2003) Influence of fixed charge density magnitude and distribution on the intervertebral disc: applications of a poroelastic and chemical electric (PEACE) model. J Biomech Eng 125:12–24
Sun DD, Leong KW (2004) A nonlinear hyperelastic mixture theory model for anisotropy, transport, and swelling of annulus fibrosus. Ann Biomed Eng 32:92–102
Wilson W, van Donkelaar CC, van Rietbergen B, Huiskes R (2005) A fibril-reinforced poroviscoelastic swelling model for articular cartilage. J Biomech 38:1195–1204
Wilson W, van Donkelaar CC, van Rietbergen B, Ito K, Huiskes R (2004) Stresses in the local collagen network of articular cartilage: a poroviscoelastic fibril-reinforced finite element study. J Biomech 37:357–366
Natarajan RN, Williams JR, Andersson GB (2004) Recent advances in analytical modeling of lumbar disc degeneration. Spine 29:2733–2741
Urban JP, Maroudas A (1981) Swelling of the intervertebral disc in vitro. Connect Tissue Res 9:1–10
Simon BR, Wu JS, Carlton MW, Kazarian LE, France EP, Evans JH, Zienkiewicz OC (1985) Poroelastic dynamic structural models of rhesus spinal motion segments. Spine 10:494–507
Biot MA (1972) Theory of finite Deformations of porous solids. Indiana Univ Math J 21:597–620
Mow VC, Kuei SC, Lai WM, Armstrong CG (1980) Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng 102:73–84
Wachtel E, Maroudas A (1998) The effects of pH and ionic strength on intrafibrillar hydration in articular cartilage. Biochim Biophys Acta 1381:37–48
Maroudas A, Wachtel E, Grushko G, Katz EP, Weinberg P (1991) The effect of osmotic and mechanical pressures on water partitioning in articular cartilage. Biochim Biophys Acta 1073:285–294
Urban JP, McMullin JF (1988) Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine 13:179–187
van der Voet (1997) A comparison of finite element codes for the solution of biphasic poroelastic problems. Proc Inst Mech Eng [H] 211:209–211
Cassidy JJ, Hiltner A, Baer E (1989) Hierarchical structure of the intervertebral disc. Connect Tissue Res 23:75–88
Iatridis JC, Kumar S, Foster RJ, Weidenbaum M, Mow VC (1999) Shear mechanical properties of human lumbar annulus fibrosus. J Orthop Res 17:732–737
Iatridis JC, Weidenbaum M, Setton LA, Mow VC (1996) Is the nucleus pulposus a solid or a fluid? Mechanical behaviors of the nucleus pulposus of the human intervertebral disc. Spine 21:1174–1184
Silver FH, Ebrahimi A, Snowhill PB (2002) Viscoelastic properties of self-assembled type I collagen fibers: molecular basis of elastic and viscous behaviors. Connect Tissue Res 43:569–580
Wang JL, Parnianpour M, ShiraziAdl A, Engin AE (1997) Failure criterion of collagen fiber: viscoelastic behavior simulated by using load control data. Theor Appl Fract Mech 27:1–12
Wilson W, van Donkelaar CC, van Rietbergen B, Ito K, Huiskes R (2005) Erratum to “Stress in the local collagen network of articular cartilage: a poroviscoelastic fibril-reinforced finite element study” and “A fibril-reinforced poroviscoelastic swelling model for articular cartilage”. J Biomech 38:2138–2140
Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M (1996) The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98:996–1003
Ferguson SJ, Steffen T (2003) Biomechanics of the aging spine. Eur Spine J 12(Suppl 2):S97–S103
Johannessen W, Elliott DM (2005) Swelling dominates function of human nucleus pulposus even with degeneration. Transactions Orthop Res Soc, vol 30, p 185, Washington, DC. Conference Proceeding
Drost MR, Willems P, Snijders H, Huyghe JM, Janssen JD, Huson A (1995) Confined compression of canine annulus fibrosus under chemical and mechanical loading. J Biomech Eng 117:390–396
Adams MA, McNally DS, Dolan P (1996) ‘Stress’ distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br 78:965–972
Edwards WT, Ordway NR, Zheng Y, McCullen G, Han Z, Yuan HA (2001) Peak stresses observed in the posterior lateral anulus. Spine 26:1753–1759
McNally DS, Adams MA, Goodship AE (1993) Can intervertebral disc prolapse be predicted by disc mechanics? Spine 18:1525–1530
Argoubi M, Shirazi-Adl A (1996) Poroelastic creep response analysis of a lumbar motion segment in compression. J Biomech 29:1331–1339
Lee KK, Teo EC (2004) Poroelastic analysis of lumbar spinal stability in combined compression and anterior shear. J Spinal Disord Tech 17:429–438
Brinckmann P, Frobin W, Hierholzer E, Horst M (1983) Deformation of the vertebral end-plate under axial loading of the spine. Spine 8:851–856
Iatridis JC, MacLean JJ, Owen JP (2005) Role of endplates in contributing to compression behaviors of motion segments and intervertebral discs. ECM VI/SRN I: Spinal motion segment: from basic science to clinical application. Davos conference proceeding
Iatridis JC, Setton LA, Foster RJ, Rawlins BA, Weidenbaum M, Mow VC (1998) Degeneration affects the anisotropic and nonlinear behaviors of human anulus fibrosus in compression. J.Biomech. 31:535–544
Bass EC, Ashford FA, Segal MR, Lotz JC (2004) Biaxial testing of human annulus fibrosus and its implications for a constitutive formulation. Ann Biomed Eng 32:1231–1242
Perie D, Iatridis JC, Demers CN, Goswami T, Beaudoin G, Mwale F, Antoniou J (2005) Assessment of compressive modulus, hydraulic permeability and matrix content of trypsin-treated nucleus pulposus using quantitative MRI. J Biomech 38:2164–2174
Acknowledgements
This research is made possible through the support of the European Union (EURODISC, Project Number QLK6-CT-2002–02582). The authors thank Donal McNally and Karen McKinlay (University of Nottingham, UK) for providing their experimental data for comparison. We would also like to thank Dr. Heiner Martin (University of Rostock, Germany) for providing the experimental anatomical measurements of a human lumbar vertebra.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Abaqus has a biphasic model of the form:
With Dirichlet boundary conditions: [u] =0 and [p] =0.
Here \({\varvec{\upsigma}}_{\mathbf{e}} \) is the effective stress, p the fluid pressure, \(\vec{q}\) the fluid flux, k the permeability and u the displacements.
The swelling behavior through the Donnan osmotic theory was included into the biphasic theory as follows:
Here μf is the chemical potential and Δπ is the osmotic pressure given by Eq. 4 with Dirichlet boundary conditions: [u] = 0 and [μf] = 0.
Rights and permissions
About this article
Cite this article
Schroeder, Y., Wilson, W., Huyghe, J.M. et al. Osmoviscoelastic finite element model of the intervertebral disc. Eur Spine J 15 (Suppl 3), 361–371 (2006). https://doi.org/10.1007/s00586-006-0110-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-006-0110-3