Abstract
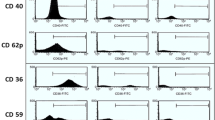
Sickle cell disease (SCD) is characterized by a hypercoagulable state as a result of multiple factors, including chronic hemolysis and the presence of circulating cell-derived microparticles (MPs). The aim of this work was to study the impact of erythrocyte-derived circulating microparticles (glycophorin A; CD235 positive) on coagulation activation and their probable role in contribution to painful crisis in SCD patients. Peripheral blood samples of 25 SCD patients during painful crisis and in steady state were studied for the presence of erythrocyte-derived MPs using flow cytometry. Estimation of D-dimer level, as a marker of coagulation activation, was done using semiquantitative assay. Thirty-six healthy individuals, age- and sex-matched, were included as a control group. Erythrocyte-derived MPs level was significantly higher in SCD patients during painful crisis compared to control group (p = 0.02), but no statistically significant difference was found between erythrocyte-derived MPs level in SCD patients in steady state compared to controls or to SCD patients during painful crisis (p = 0.3 and 0.49, respectively). D-dimer level was higher in SCD patients both during crisis and in steady state compared to controls (p < 0.001). SCD during painful crisis is associated with increased levels of erythrocyte-derived MPs and D-dimer which may contribute to the hypercoagulable state observed in such group of patients.


Similar content being viewed by others
References
Adam SS, Key NS, Greenberg CS (2009) D-dimer antigen: current concepts and future prospects. Blood 113(13):2878–2887
Allan D, Limbrick AR, Thomas P, Westerman MP (1982) Release of spectrin free spicules on reoxygenation of sickled erythrocytes. Nature 295:612–613
Ardoin SP, Shanahan JC, Pisetsky DS (2007) The role of microparticles in inflammation and thrombosis. Scand J Immunol 66:159–165
Ataga KI, Key NS (2007) Hypercoagulability in sickle cell disease: new approaches to an old problem. Hematol (Am Soc Hematol Educ Program) 91–96
Austin H, Key NS, Benson JM, Lally C, Dowling NF, Whitsett C et al (2007) Sickle cell trait and the risk of venous thromboembolism among blacks. Blood 110:908–912
Ballas SK, Lusardi M (2005) Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol 79:17–25
Berckmans RJ, Nieuwland R, Kraan MC, Schaap M, Pots D, Smeets T et al (2005) Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res Ther 7:R536–R544
Bunn H, Forget BG (1986) Hemoglobin: molecular, genetic and clinical aspects. WB Saunders, Philadelphia, PA, USA
Camus SM, Gausserès B, Bonnin P, Loufrani L, Grimaud L, Charue D et al (2012) Erythrocyte microparticles can induce kidney vaso-occlusions in a murine model of sickle cell disease. Blood 120(25):5050–5058
Dover GJ, Platt OS (1998) Sickle Cell Disease. In: Nathan and Oski’s Hematology of infancy and childhood, DG Nathan and SH Orkin (eds), 5th Edition, WB Saunders Company Volume 1.
Edelstein SJ, Telford JN, Crépeau RH (1973) Structure of fibers of sickle cell hemoglobin. Proc Natl Acad Sci U S A 70:1104–1107
Fontana V, Jy W, Ahn ER, Dudkiewicz P, Horstman LL, Duncan R et al (2008) Increased procoagulant cell-derived microparticles (CMP) in splenectomized patients with ITP. Thromb Res 122:599–603
Horne MK, Cullinane AM, Merryman PK, Hoddeson EK (2006) The effect of red blood cells on thrombin generation. Br J Haematol 133:403–408
Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM (2005) Membrane microparticles: two sides of the coin. Physiology (Bethesda) 20:22–27
Kriebardis A, Antonelou M, Stamoulis K, Papassideri I (2012) Cell-derived microparticles in stored blood products: innocent-bystanders or effective mediators of post-transfusion reactions. Blood Transfus 10(Suppl 2):s25–s38
Kuypets FA, Lewis RA, Hua M, Schott MA, Discher D, Ernst JD et al (1996) Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood 87:1179–1187
Mann KG (1999) Biochemistry and physiology of blood coagulation. Thromb Haemost 82:165–174
Mezentsev A, Merks RM, O’Riordan E, Chen J, Mendelev N, Goligorsky MS et al (2005) Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol 289:H1106–H1114
Nébor D, Romana M, Santiago R, Vachiery N, Picot J, Broquere C et al (2013) Fetal hemoglobin and hydroxycarbamide modulate both plasma concentration and cellular origin of circulating microparticles in sickle cell anemia children. Haematologica 98(6):862–867
Neidlinger NA, Hirvela ER, Skinner RA (2005) Postinjury serum secretory phospholipase A2 correlates with hypoxemia and clinical status at 72 hours. J Am Coll Surg 200:173–178
Neidlinger NA, Larkin SK, Bhagat A (2006) Hydrolysis of phosphatidylserine exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction. J Biol Chem 281:775–781
Piccin A, Murphy WG, Smith OP (2007) Circulating microparticles: pathophysiology and clinical implications. Blood Rev 21:157–171
Rubin O, Crettaz D, Canellini G, Tissot JD, Lion N (2008) Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sang 95:288–297
Setty BN, Rao AK, Stuart MJ (2001) Thrombophilia in sickle cell disease: the red cell connection. Blood 98:3228–3233
Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N et al (2003) Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood 102:2678–2683
Stuart MJ, Nagel RL (2004) Sickle-cell disease. Lancet 364:1343–1360
Stuart MJ, Setty BN (2001) Hemostatic alterations in sickle cell disease: relationships to disease pathophysiology. Pediatr Pathol Mol Med 20:27–46
Tait JF, Gibson D (1994) Measurement of membrane phospholipid asymmetry in normal and sickle-cell erythrocytes by means of annexin V binding. J Lab Clin Med 123:741–748
Tantawy AA, Adly AA, Ismail EA, Habeeb NM, Farouk A (2012) Circulating platelet and erythrocyte microparticles in young children and adolescents with sickle cell disease: relation to cardiovascular complications. Platelets 18(1):167–178
Tissot JD, Rubin O, Canellini G (2010) Analysis and clinical relevance of microparticles from red blood cells. Curr Opin Hematol 17:571–577
van Beers E, Schaap M, Berckmans R, Nieuwland R, Sturk A, van Doormaal F et al (2009) Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica 94(11):1513–1519
Westerman M, Pizzey A, Hirschman J, Cerino M, Weil- Weiner Y, Ramotar P et al (2008) Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. Br J Haematol 142:126–135
Wun T, Paglieroni T, Rangaswami A, Franklin PH, Welborn J, Cheung A et al (1998) Platelet activation in patients with sickle cell disease. Br J Haematol 100:741–749
Zwaal RF, Schroit AJ (1997) Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 89:1121–1132
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zayed, R.A., El-Ghamrawi, M., Alwakeel, H.A. et al. Impact of circulating erythrocyte-derived microparticles on coagulation activation in sickle cell disease. Comp Clin Pathol 24, 1123–1128 (2015). https://doi.org/10.1007/s00580-014-2045-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-014-2045-0