Abstract
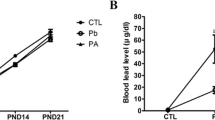
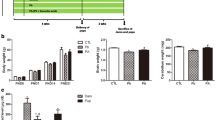
Lead is a well-known neurotoxin that affects the developing central nervous system and may potentially inhibit neurogenesis in adults. We investigated the effect of ascorbic acid and garlic extract against lead-induced neurotoxicity in developing rat dentate gyrus. Female Wistar rats were divided randomly into five groups: lead-treated (L; 1,500 ppm lead acetate in drinking water) group, lead plus ascorbic acid-treated (L + AA; 500 mg/kg, ip) group, lead plus garlic juice-treated (L + G; 1 ml /100 g BW, gavage) group, sham group (sh), and controls. All treatments were administered to female rats during pregnancy and lactation. At the end of the treatment, dentate gyrus neurogenesis were determined using Doublecortin (DCX) immunohistochemistry in the hippocampus of 50-day-old male pups. DCX-positive cells in the dentate gyrus were counted and compared between the groups. Lead exposure caused a significant increase in blood and brain lead concentration vs. control (P < 0.001); whereas, co-administration of ascorbic acid or garlic + lead was effective in reducing blood and brain lead levels (P < 0.01). The number of DCX-positive cells in the dentate gyrus of the lead-exposed group was significantly lower, when compared with controls. A statistically significant increase in number of DCX-positive cells in ascorbic acid and garlic groups compared with lead-exposed rats was noted (P < 0.05). This study provides evidence of the beneficial role of ascorbic acid and garlic in early status of dentate gyrus neurogenesis in rats against lead exposure.


Similar content being viewed by others
References
Ahamed M, Siddiqui MKJ (2007) Low level lead exposure and oxidative stress: current opinions. Clin Chim Acta 383:57–64
Antonio-Garcia MT, Masso-Gonzalez EL (2008) Toxic effects of perinatal lead exposure on the brain of rats: involvement of oxidative stress and the beneficial role of antioxidants. Food Chem Toxicol 46(6):2089–2095
Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, Waternaux C (1991) Low-level lead exposure and children's cognitive function in the preschool years. Pediatrics 87(2):219–227
Bressler J, Kim KA, Chakraborti T, Goldstein G (1999) Molecular mechanisms of lead neurotoxicity. Neurochem Res 24(4):595–600
Burdette LJ, Goldstein R (1986) Long-term behavioral and electrophysiological changes associated with lead exposure at different stages of brain development in the rat. Dev Brain Res 29:101–110
Canfield RL, Henderson CR Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP (2003) Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med 348(16):1517–1526
Chang BJ, Jang BJ, Son TG, Cho IH, Quan FS, Choe NH, Nahm SS, Lee JH (2012) Ascorbic acid ameliorates oxidative damage induced by maternal low-level lead exposure in the hippocampus of rat pups during gestation and lactation. Food Chem Toxicol 50(2):104–108
Chung LY (2006) The antioxidant properties of garlic compounds: allyl cysteine, alliin, allicin, and allyl disulfide. J Med Food 9(2):205–213
Couloures K, Vasan R (2011) Prenatal lead poisoning due to maternal exposure results in developmental delay. Pediatr Int 53(2):242–244
Dribben WH, Creeley CE, Farber N (2011) Low-level lead exposure triggers neuronal apoptosis in the developing mouse brain. Neurotoxicol Teratol 33(4):473–480
Ennever FK (1994) Metals. In: Hayes AW (ed) Principles and method of toxicology. Raven Press, USA, New York, pp 417–446
Flora SJ, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 128(4):501–523
Gilbert ME, Kelly ME, Samsam TE, Goodman JH (2005) Chronic developmental lead exposure reduces neurogenesis in adult rat hippocampus but does not impair spatial learning. Toxicol Sci 86(2):365–374
Goyer RA (1990) Transplacental transport of lead. Environ Health Perspect 89:101–105
Goyer RA (1996) Results of lead research: prenatal exposure and neurological consequences. Environ Health Perspect 104(10):1050–1054
Goyer RA, Cherian MG (1979) Ascorbic acid and EDTA treatment of lead toxicity in rats. Life Sci 24(5):433–438
Gurer H, Ercal N (2000) Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med 29(10):927–945
Han JM, Chang BJ, Li TZ, Choe NH, Quan FS, Jang BJ, Cho IH, Hong HN, Lee JH (2007) Protective effects of ascorbic acid against lead-induced apoptotic neurodegeneration in the developing rat hippocampus in vivo. Brain Res 1185:68–74
Howaed CV, Reed MG (2005) Unbiased stereology. BIOS Scientific Publisher Ltd, Oxford, UK
Hsu PC, Guo YL (2002) Antioxidant nutrients and lead toxicity. Toxicology 180(1):33–44
Iciek M, Kwiecien I, Wlodek L (2009) Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen 50(3):247–265
Jaako-Movits K, Zharkovsky T, Romantchik O, Jurgenson M, Merisalu E, Heidmets LT, Zharkovsky A (2005) Developmental lead exposure impairs contextual fear conditioning and reduces adult hippocampal neurogenesis in the rat brain. Int J Dev Neurosci 23(7):627–635
Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16(6):2027–2033
Leelarungrayub N, Rattanapanone V, Chanarat N, Gebicki JM (2006) Quantitative evaluation of the antioxidant properties of garlic and shallot preparations. Nutrition 22(3):266–274
Lockitch G (1993) Perspectives on lead toxicity. Clin Biochem 26:371–381
Mansouri MT, Naghizadeh B, Lopez-Larrubia P, Cauli O (2013) Behavioral deficits induced by lead exposure are accompanied by serotonergic and cholinergic alterations in the prefrontal cortex. Neurochem Int 62(3):232–239
Massadeh AM, Al-Safi SA, Momani IF, Alomary AA, Jaradat QM, AlKofahi AS (2007) Garlic (Allium sativum L.) as a potential antidote for cadmium and lead intoxication: cadmium and lead distribution and analysis in different mice organs. Biol Trace Elem Res 120(1–3):227–234
Moreira EG, Vassilieff I, Vassilieff VS (2001) Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol 23(5):489–495
Murphy KJ, Regan CM (1999) Low-level lead exposure in the early postnatal period results in persisting neuroplastic deficits associated with memory consolidation. J Neurochem 72(5):2099–2104
Okada Y, Tanaka K, Fujita I, Sato E, Okajima H (2005) Antioxidant activity of thiosulfinates derived from garlic. Redox Rep 10(2):96–102
Oommen S, Anto RJ, Srinivas G, Karunagaran D (2004) Allicin (from garlic) induces caspase-mediated apoptosis in cancer cells. Eur J Pharmacol 485(1–3):97–103
Rogan WJ, Ware JH (2003) Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. J Pediatr 143(5):687–688
Schneider JS, Anderson DW, Wade TV, Smith MG, Leibrandt P, Zuck L, Lidsky TI (2005) Inhibition of progenitor cell proliferation in the dentate gyrus of rats following post-weaning lead exposure. Neurotoxicology 26(1):141–145
Senapati SK, Dey S, Dwivedi SK, Swarup D (2001) Effect of garlic (Allium sativum L.) extract on tissue lead level in rats. J Ethnopharmacol 76(3):229–232
Shahsavani D, Baghshani H, Alishahi E (2011) Efficacy of allicin in decreasing lead (Pb) accumulation in selected tissues of lead-exposed common carp (Cyprinus carpio). Biol Trace Elem Res 142(3):572–580
Shalan MG, Mostafa MS, Hassouna MM, El-Nabi SE, El-Refaie A (2005) Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicology 206(1):1–15
Shan G, Tang T, Zhang X (2009) The protective effect of ascorbic acid and thiamine supplementation against damage caused by lead in the testes of mice. J Huazhong Univ Sci Technolog Med Sci 29(1):68–72
Sharma V, Sharma A, Kansal L (2010) The effect of oral administration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem Toxicol 48(3):928–936, 2
Toscano CD, Guilarte TR (2005) Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev 49(3):529–554
Verina T, Rohde CA, Guilarte TR (2007) Environmental lead exposure during early life alters granule cell neurogenesis and morphology in the hippocampus of young adult rats. Neuroscience 145(3):1037–1047
Wang C, Liang J, Zhang C, Bi Y, Shi X, Shi Q (2007) Effect of ascorbic acid and thiamine supplementation at different concentrations on lead toxicity in liver. Ann Occup Hyg 51(6):563–569
Winneke G, Kramer U (1997) Neurobehavioral aspects of lead neurotoxicity in children. Cent Eur J Public Health 5(2):65–69
Acknowledgments
This study was supported by grant no. 900992 from vice chancellor for research, Mashhad University of Medical Sciences, Mashhad, Iran. In addition, the authors would like to thank Mrs. Motejaded for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alipour, F., Bideskan, A.E., Fazel, A. et al. Protective effects of ascorbic acid and garlic extract against neurogenesis inhibition caused by developmental lead exposure in the dentate gyrus of rat. Comp Clin Pathol 23, 1681–1687 (2014). https://doi.org/10.1007/s00580-014-1895-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-014-1895-9