Abstract
The acute phase response is a nonspecific inflammatory reaction of the host that occurs shortly after any tissue injury. The response includes changes in the concentration of plasma proteins called acute phase proteins (APPs). Calf diarrhea is an important disease that occurs in association with the interaction of various infectious agents and calf susceptibility. The economic losses is associated with death loss and treatment costs, reduction of live weight gain, and reduction of productive life span, which may be considerable. The aim of the present study was to identify relationships among APPs in calves with diarrhea in the different clinical features. Holstein calves (50) within 1 day to 4 months old with signs of diarrhea and healthy calves (40) with similar age and sex were selected. Standard clinical examinations and also dehydration degree were carried out on each calf and were recorded. Calves with clinical signs of diarrhea were divided in different groups based on the severity of the clinical findings, fever and degree of dehydration. Blood samples were taken from the jugular vein from all calves into vacutainers containing ethylenediaminetetraacetic acid (EDTA) for separating plasma and without EDTA for serum biochemical analysis. APP [haptoglobin (Hp), serum amyloid A (SAA), fibrinogen (Fib), and ceruloplasmin (Cp)] concentrations were measured using validated standard methods. The results indicated a significant increases in APPs in diarrheic calves which was most obvious in Hp and SAA (P < 0.001). Calves with severe clinical signs of diarrhea had a significant increases in their Hp and SAA (P < 0.001) compared to calves with moderate or without systemic clinical signs. Diarrheic calves with fever compared to diarrheic calves without fever had a significant increases in their Hp and SAA (P < 0.01). Also, diarrheic calves with severe dehydration compared to diarrheic calves with mild and moderate dehydration had significant increases in Hp and SAA (P < 0.05), and these parameters (Hp, SAA, Fib, and Cp) among calves with mild and moderate dehydration had no significant changes. Our results indicated that monitoring the APP responses in diarrheic calves with different clinical signs could be useful as prognostic tools and facilitate treatment decisions.
Similar content being viewed by others
Introduction
Neonatal diarrhea is a major source of economic loss to the cattle industry and the leading cause of calf mortality in most countries. Financial losses arise not only from mortality, but also from the cost of medication and labor needed to treat sick calves (Constable et al. 2001). The main etiologic agents of diarrhea in dairy calves are Escherichia coli K99 (F5), Salmonella spp., rotavirus, coronavirus, and Cryptosporidium parvum. These pathogens cause specific enteric infections, resulting in secretory or malabsorptive diarrhea, along with inflammation of the intestinal epithelium (Foster and Smith 2009). Sickness behavior is an integrated response to infection and inflammation, involving characteristic behavioral and physiological changes including loss of appetite, somnolence, increased thermoregulatory behavior, and reduced social activity (Hart 1988; Todd et al. 2010).
Animal health can be defined as the absence of disease determined by clinical examinations combined with various diagnostic tests. However, such clinical examinations may suffer from a lack of reproducibility (Petersen et al. 2004). The acute phase response is the reaction of the animal to disturbances in its homeostasis caused by infection, tissue injury, neoplastic growth, or immunological disorders. It is characterized by alterations in the concentrations of a variety of hepatocyte-derived acute phase proteins (APPs) in the blood, fever and endocrinological, metabolic, immunological, and neurological changes (Pfeffer et al. 1993). The acute phase proteins (APPs) are blood proteins that can be used to assess the innate immune system’s systemic response to infection, inflammation, or trauma (Murata et al. 2004; Petersen et al. 2004; Cerón et al. 2005). APPs may provide an alternative means of monitoring animal health. An increased focus on the application of APP for this purpose has recently been developed (Skinner et al. 1991). Due to a relatively short half-life in serum and high response in diseased animals, APP serum responses constitute a valid measure of a systemic response to an initiating stimulus at the time of blood sampling. Important points to consider before using APP as objective and nonspecific markers of animal health are the possible influence of environmental factors, handling, and other types of stress in the absence of disease. These biomarkers are highly sensitive indicators of inflammation but lack specificity, and there are major species differences in the APP response (Eckersall and Bell 2010). Measurement of the acute phase proteins is a potentially useful clinical tool in veterinary medicine, but further studies are required to assess their responses in different pathological processes according to the species (Kent 1992). The haptoglobin (Hp), ceruloplasmin (Cp), and fibrinogen (Fib) concentrations have been reported to increase in many infections and inflammatory conditions in ruminants (Conner et al. 1988, 1989; Skinner et al. 1991; Pfeffer et al. 1993; Wittum et al. 1996; Gånheim et al. 2003). Hp and serum amyloid A (SAA) are important bovine APPs, which increase in serum, for example, during viral and bacterial diseases (Murata et al. 2004; Petersen et al. 2004), but are absent, or present, in very low levels, in healthy animals (Godson et al. 1996; Heegaard et al. 2000; Gånheim et al. 2003). Moreover, subclinical inflammatory disorders can induce increase in APP concentrations (Karreman et al. 2000). The concentration of Fib is increased in the plasma of animals with inflammatory disorders (McSherry and Horney 1970) and has been used for many years to evaluate inflammatory disease in cattle (Eckersall and Conner 1988). Lungworm infection (Gånheim et al. 2004) and stress due to poor housing (Alsemgeest et al. 1995), weaning, transportation, and mixing (Arthington et al. 2003) can also result in increased APP concentrations in cattle. APPs have been considered both as potential indicators of disease and well-being in individual animals and as indicators of herd health (Alsemgeest et al. 1994; Murata et al. 2004; Petersen et al. 2004). Studies show that SAA (Karreman et al. 2000) and Hp (Saini et al. 1998) have been found useful in herd screenings to identify cows with inflammatory diseases. At the herd level, APP might be useful for determining where the spread of the disease is taking place (age group, part of the production system), by providing information about the prevalence of ongoing clinical and subclinical infections indicated by the high serum concentration of selected APP (Petersen et al. 2004) and by serving as a prognostic tool, with the magnitude and duration of the acute phase response reflecting the severity of infection (Skinner et al. 1991; Petersen et al. 2004).
The aim of this study was to describe the APP responses in calf diarrhea complex with different clinical features.
Materials and methods
Animals
Holstein calves (50) within 1 day to 4 months old with signs of diarrhea and healthy calves (40) with similar age as case and control groups from the same management, respectively, were selected.
Clinical examination
A clinical examination was conducted in calves with sign of diarrhea without any other diseases in which heart rate and respiratory rate were determined, rectal temperature was measured. Individual calf fecal consistency scores were assigned using a four-point scale (1 = normal, firm stool; 2 = soft, does not hold form; 3 = runny, spreads easily; 4 = liquid, devoid of solid matter), as described by Larson et al. (1977). Diarrhea was defined as a fecal score greater than 2. Fecal scores of 3 and 4 were described as mild and severe diarrhea, respectively. At the naturally occurring onset of diarrhea, defined as the first occurrence of a fecal score greater than 2, the calves were enrolled in the study. Rectal temperatures above 39.5 were recorded as febrile calves, presence or absence of suckling reflex were determined, and recumbence calves and shock signs, such as rapid heart rate, cold extremities, and weakness, were recorded. Also, dehydration degree were carried out on each calf and were assessed by “tenting” the skin of the lateral portion of the cervical region and by measuring the time required for the skin fold to return to normal based on Radostits et al. (2007). Calves with clinical signs of diarrhea were divided in different groups based on the severity of the clinical findings, fever, and degree of dehydration.
Blood sampling
Blood samples were taken from the jugular vein from all calves (control and case) in the same time in the first day of the disease into vacutainers containing ethylenediaminetetraacetic acid (EDTA) for separating plasma and measuring hematological parameters and without EDTA for serum biochemical analysis. The sera and plasma are separated by centrifugation at 750 × g for 15 min; the plasma and serum was transferred to a plastic vial, capped, and stored at -20°C until analyzed. The animals had not been treated for disease prior to sampling.
Measurements
APP concentrations were measured using validated standard methods.
Hp determination
Hp was measured according to prevention of the peroxidase activity of hemoglobin, which is directly proportional to the amount of Hp (Tietz et al. 2006). The analytical sensitivity of this test in serum has been determined as 15.6 mg/l for Hp by the manufacturer (Tridelta Development, Wicklow, Ireland).
SAA determination
SAA was measured by a solid phase sandwich enzyme-linked immunosorbent assay (ELISA; Tietz et al. 2006). The analytical sensitivity of this test in serum has been determined as 0.3 mg/l for SAA by the manufacturer (Tridelta Development).
Cp determination
Cp was measured using the method of Sunderman and Nomoto (1970).
Fib determination
Fib was measured by the precipitation refractory method as described by Thrall et al. (2004)
Statistical analysis
Data were presented as mean ± standard error (SE) and median. Due to inequality of variances and nonnormal distribution of data, to investigate any important changes in study groups, nonparametric Kruskal–Wallis and Mann–Whitney U test were used for statistical comparisons. Group differences were considered statistically significant at P < 0.05.
Results
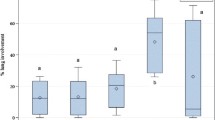
Blood concentrations of APPs in calves with diarrhea and healthy calves are presented as mean ± SE and median in Table 1, also APPs in calves with diarrhea according to different clinical signs are presented as mean ± SE and median in Table 2. There was a significant difference in Hp, SAA, Cp, and Fib between healthy and diseased calves which was most obvious in Hp and SAA (P < 0.001). Calves with severe clinical signs of diarrhea had significant increases in their Hp and SAA (P < 0.001) as compared to calves with moderate or without systemic clinical signs. Diarrheic calves with fever had significant increases in their Hp and SAA as compared to diarrheic calves without fever (P < 0.01). Also, diarrheic calves with severe dehydration compared to diarrheic calves with mild and moderate dehydration had significant increases in Hp and SAA (P < 0.05), and these parameters among calves with mild and moderate dehydration had no significant changes.
Discussion
In bovines, two major serum APPs proteins have been recognized: SAA (Horadagoda et al. 1999) and Hp (Eckersall and Conner 1988). They have been used to evaluate inflammatory conditions in clinical or experimental cattle studies (Alsemgeest et al. 1994; Heegaard et al. 2000). A large proportion of diarrheic calves in our study had elevated concentrations of one or more APPs which were most obvious in Hp and SAA. Hp has been reported to be a useful indicator of bovine bacterial infections (Eckersall 2000). Under normal conditions, Hp is absent or present in very low concentrations in serum, ranging from 0.05 to 0.10 g/l, but the concentration may increase 50–100 times after infection or inflammation (Conner et al. 1989; Skinner et al. 1991; Pfeffer et al. 1993; Wittum et al. 1996). In our study, the mean concentration of Hp in diarrheic calves was 270 mg/l, which was significantly higher than healthy calves (90 mg/l). In cattle, an increased SAA serum and plasma concentration has been found following experimentally induced (Bremner 1964; Conner et al. 1988) and naturally occurring inflammation (Alsemgeest et al. 1994) as well as experimental and natural infections. The SAA response during viral respiratory diseases is well described (Heegaard et al. 2000; Gånheim et al. 2003). After intratracheal inoculation with Pasteurella multocida, the SAA concentration increased (Horadagoda et al. 1994).
In feedlot cattle with clinical respiratory tract disease, a high but variable Hp response was observed (Wittum et al. 1996). No correlation between Hp serum concentration and rectal temperature or pathological lesions in the lungs was found, but lower serum concentration was observed in cattle treated with antibiotics. Hp has also been associated with bacterial contamination of the uterus and delayed uterine involution (Sheldon et al. 2001). As for Hp, the SAA response is of longer duration in coinfections with virus and bacteria when compared to pure virus infections (Gånheim et al. 2003). Some authors indicated that the magnitude and the duration of the response correlated well with the severity of the clinical signs (Heegaard et al. 2000; Gånheim et al. 2003) Calves with severe clinical signs of diarrhea in our study had significant increases in their Hp and SAA compared to calves with moderate or without systemic clinical signs. Diarrheic calves with fever had significant increases in their Hp and SAA compared to diarrheic calves without fever.
The increases in APPs in diarrheic calves were well correlated with the clinical findings in this group. The difference between the diarrheic and healthy calves was most obvious for Hp and SAA indicating that may be the most useful predictive APPs and is in agreement with Carter et al. (2002) who concluded that analysis of serum Hp was a better tool for discrimination between calves that became ill and those that did not, as compared to other APPs. Cp is less commonly measured than Hp, but increased in our diarrheic calves. Cp has been identified as an APP in many species (Feldman et al. 2000). In comparison to SAA and Hp, Cp and Fib were less sensitive to change in diarrheic calves in our study. The application of Cp to diagnosis remains less common than that of other APP, but there have been a number of studies confirming that this ferroxidase is an indicator of infection in cattle (Conner et al. 1988, 1989; Chassagne et al. 1998; Sheldon et al. 2001). The sensitivity of SAA was more than Hp, Cp, and Fib for inflammatory reactions of bovine theileriosis (Nazifi et al. 2009). APPs increase when a disease developed and decrease in the recovery stage (Nazifi et al. 2009). SAA, in comparison with other APPs, is a more suitable diagnostic indicator of inflammatory process in bovine theileriosis (Nazifi et al. 2009). SAA is reported to be more sensitive to stimulation (Horadagoda et al. 1999; Heegaard et al. 2000), and as an increase can be induced also by other factors than disease, such as stress (Alsemgeest et al. 1995), it may be less suitable as an indicator of health problems. APPs, produced during the early inflammatory response, represent a potentially useful systemic marker of bacterial infection. In a calf model of experimentally induced Salmonella infection, serum Hp levels increase within 3 days of challenge. These increases correlate with the development of clinical symptoms of infection, diarrhea, and rectal temperature. Therefore, determination of serum Hp levels may provide a useful marker of Salmonella infection in young calves (Deignan et al. 2000). Alsemgeest et al. (1994) reported that Serum Hp in combination with other APP seemed to be a promising indicator of disease on a herd level basis. Gånheim et al. (2004) investigated the potential of APP (Hp, Fib, and SAA) as an objective tool to evaluate animal health and management in two groups of calves with different clinical health status and reported the largest difference in number of days per calf with supra-normal concentrations to occur for serum Hp, suggesting this to be the most useful APP to measure. Serum Hp, when combined with rectal temperature, may be a valuable parameter in herd level diagnostics at least in heifers(Svensson et al. 2007).
The results of the present study support the usefulness of APP measurement in monitoring animal health and support the proposals made by other authors (Alsemgeest et al. 1994; Murata et al. 2004; Petersen et al. 2004). Like rectal temperature, APP levels are not suitable for establishing a specific diagnosis but can provide objective information about the extent of ongoing lesions in individual animals (Petersen et al. 2004). Our results indicated that monitoring the APPs responses in diarrheic calves with different clinical signs could be useful as prognostic tools and facilitate treatment decisions.
References
Alsemgeest S, Kalsbeek H, Wensing T, Koeman J, Van Ederen A, Gruys E (1994) Concentrations of serum amyloid-A (SAA) and haptoglobin (HP) as parameters of inflammatory diseases in cattle. Vet Q 16:21
Alsemgeest S, Lambooy I, Wierenga H, Dieleman S, Meerkerk B, Van Ederen A, Niewold TA (1995) Influence of physical stress on the plasma concentration of serum amyloid-A (SAA) and haptoglobin (Hp) in calves. Vet Q 17:9
Arthington J, Eicher S, Kunkle W, Martin F (2003) Effect of transportation and commingling on the acute-phase protein response, growth, and feed intake of newly weaned beef calves. J Anim Sci 81:1120
Bremner K (1964) Studies on haptoglobin and haemopexin in the plasma of cattle. Aust J Exp Biol Med Sci 42:643–656
Carter JN, Meredith GL, Montelongo M, Gill DR, Krehbiel CR, Payton ME, Confer AW (2002) Relationship of vitamin E supplementation and antimicrobial treatment with acute-phase protein responses in cattle affected by naturally acquired respiratory tract disease. Am J Vet Res 63:1111–1117
Cerón JJ, Eckersall PD, Martínez Subiela S (2005) Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet Clin Pathol 34:85–99
Chassagne M, Barnouin J, Chacornac J (1998) Biological predictors for early clinical mastitis occurrence in Holstein cows under field conditions in France. Prev Vet Med 35:29–38
Conner J, Eckersall P, Wiseman A, Aitchison T, Douglas T (1988) Bovine acute phase response following turpentine injection. Res Vet Sci 44:82
Conner J, Eckersall P, Wiseman A, Bain R, Douglas T (1989) Acute phase response in calves following infection with Pasteurella haemolytica, Ostertagia ostertagi and endotoxin administration. Res Vet Sci 47:203
Constable P, Thomas E, Boisrame B (2001) Comparison of two oral electrolyte solutions for the treatment of dehydrated calves with experimentally-induced diarrhoea. Vet J 162:129–140
Deignan T, Alwan A, Kelly J, McNair J, Warren T, O’Farrelly C (2000) Serum haptoglobin: an objective indicator of experimentally-induced Salmonella infection in calves. Res Vet Sci 69:153–158
Eckersall P (2000) Recent advances and future prospects for the use of acute phase proteins as markers of disease in animals. Rev Méd Vét 151:577–584
Eckersall P, Bell R (2010) Acute phase proteins: biomarkers of infection and inflammation in veterinary medicine. Vet J 185:23–27
Eckersall P, Conner J (1988) Bovine and canine acute phase proteins. Vet Res Commun 12:169–178
Feldman BF, Zinkl JG, Schalm OW (2000) Schalm’s veterinary hematology. Wiley-Blackwell
Foster D, Smith GW (2009) Pathophysiology of diarrhea in calves. Veterinary clinics of North America. Food Anim Pract 25:13–36
Gånheim C, Hulten C, Carlsson U, Kindahl H, Niskanen R, Waller KP (2003) The acute phase response in calves experimentally infected with bovine viral diarrhoea virus and/or Mannheimia haemolytica. J Vet Med Ser B 50:183–190
Gånheim C, Höglund J, Waller KP (2004) Acute phase proteins in response to Dictyocaulus viviparus infection in calves. Acta Vet Scand 45:1–8
Godson DL, Campos M, Attah-Poku SK, Redmond MJ, Cordeiro DM, Sethi MS, Harland RJ, Babiuk LA (1996) Serum haptoglobin as an indicator of the acute phase response in bovine respiratory disease. Vet Immun Immunopathol 51:277–292
Hart BL (1988) Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–137
Heegaard PMH, Godson DL, Toussaint MJM, Tjørnehøj K, Larsen LE, Viuff B, Rønsholt L (2000) The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Vet Immun Immunopathol 77:151–159
Horadagoda A, Eckersall P, Hodgson J, Gibbs H, Moon G (1994) Immediate responses in serum tnf [alpha] and acute phase protein concentrations to infection with Pasteurella haemolytica A1 in calves. Res Vet Sci 57:129–132
Horadagoda N, Knox K, Gibbs H, Reid S, Horadagoda A, Edwards S, Eckersall P (1999) Acute phase proteins in cattle: discrimination between acute and chronic inflammation. Vet Rec 144:437
Karreman H, Wentink G, Wensing T (2000) Using serum amyloid A to screen dairy cows for sub-clinical inflammation. Vet Q 22:175–178
Kent J (1992) Acute phase proteins: their use in veterinary diagnosis. Br Vet J (UK)
Larson L, Owen F, Albright J, Appleman R, Lamb R, Muller L (1977) Guidelines Toward more uniformity in measuring and reporting calf experimental data 1. J Dairy Sci 60:989–991
McSherry B, Horney F (1970) Plasma fibrinogen levels in normal and sick cows. Can J Comp Med 34:191
Murata H, Shimada N, Yoshioka M (2004) Current research on acute phase proteins in veterinary diagnosis: an overview. Vet J 168:28–40
Nazifi S, Razavi S, Esmailnejad Z, Gheisari H (2009) Study on acute phase proteins (haptoglobin, serum amyloid A, fibrinogen, and ceruloplasmin) changes and their diagnostic values in bovine tropical theileriosis. Parasitol Res 105:41–46
Petersen HH, Nielsen JP, Heegaard PMH (2004) Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res 35:163–187
Pfeffer A, Rogers K, O’keeffe L, Osborn P (1993) Acute phase protein response, food intake, liveweight change and lesions following intrathoracic injection of yeast in sheep. Res Vet Sci 55:360–366
Radostits OM, Gay CC, Hinchcliff KW, Constable PD (2007) Veterinary Medicine: A textbook of the diseases of cattle, horses, sheep, pigs and goats. Saunders Elsevier Philadelphia, PA
Saini P, Riaz M, Webert D, Eckersall P, Young C, Stanker L, Chakrabarti E, Judkins J (1998) Development of a simple enzyme immunoassay for blood haptoglobin concentration in cattle and its application in improving food safety. Am J Vet Res 59:1101
Sheldon I, Noakes D, Rycroft A, Dobson H (2001) Acute phase protein responses to uterine bacterial contamination in caftle after calving. Vet Rec 148:172
Skinner J, Brown R, Roberts L (1991) Bovine haptoglobin response in clinically defined field conditions. Vet Rec 128:147
Sunderman FW Jr, Nomoto S (1970) Measurement of human serum ceruloplasmin by its p-phenylenediamine oxidase activity. Clin Chem 16:903
Svensson C, Liberg P, Hultgren J (2007) Evaluating the efficacy of serum haptoglobin concentration as an indicator of respiratory-tract disease in dairy calves. Vet J 174:288–294
Thrall MA, Baker DC, Lassen ED (2004) Veterinary hematology and clinical chemistry. Wiley-Blackwell
Tietz NW, Burtis CA, Ashwood ER, Bruns DE (2006) Tietz textbook of clinical chemistry and molecular diagnostics. Elsevier Saunders
Todd C, Millman S, McKnight D, Duffield T, Leslie K (2010) Nonsteroidal anti-inflammatory drug therapy for neonatal calf diarrhea complex: effects on calf performance. J Anim Sci 88:2019
Wittum T, Young C, Stanker L, Griffin D, Perino L, Littledike E (1996) Haptoglobin response to clinical respiratory tract disease in feedlot cattle. Am J Vet Res 57:646
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajimohammadi, A., Nazifi, S., Ansari-Lari, M. et al. Identifying relationships among acute phase proteins (haptoglobin, serum amyloid A, fibrinogen, ceruloplasmin) and clinical findings in dairy calf diarrhea. Comp Clin Pathol 22, 227–232 (2013). https://doi.org/10.1007/s00580-011-1390-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-011-1390-5