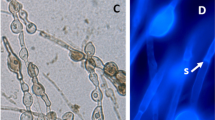
Abstract
The extramatrical mycelia of Suillus bovinus, Rhizopogon luteolus and R. vinicolor, all examples of hydrophobic (ho), mat-forming mycorrhizal fungi, were examined while associated with their hosts in the unsterilized rhizoscope, and efforts were made to produce and examine similar structures in vitro. Comparisons were made with four hydrophilic (hi) mycorrhizal fungi, Thelephora terrestris, Cenococcum geophilum, Laccaria laccata and Hebeloma crustuliniforme. The ho fungi formed linear structures (coarse, rhizomorph-like cords, with vessels in the center) and fans, both in the rhizoscope and in vitro. The same was seen in mycorrhizal mycelia in forest soils. These cords did not themselves give rise to the fans peripherally, and were not proper rhizomorphs, but were created continuously from single exploring air hyphae in the preexisting fan. Thus the ho exploring hyphae aggregated into strands, which grew in thickness only when no suitable, exploitable substrate was found. The assembly of hyphae creating ho cords was seen in the air as well as on inert hydrophilic (glass) or hydrophobic (plastic) surfaces, but never in water. It is hypothesized that the ho cell wall surface glues hyphae together while cords are formed. Water disturbed strands and mantles already formed. The ho exploring hyphae could also create ho mycelial patches (as in a mat) at the water-air interface of a number of substrates. The periphery of these patches seemed to be composed of shorter exploiting hyphae penetrating different water-soaked substrates. Exploring, aerial hyphal tips of the ho fungi were shown to “excrete” water droplets from openings in the ho cell wall surface, both in vitro and in the rhizoscope. In the rhizoscope, droplet excretion was apparently directly governed by photosynthesis in the shoot of the seedling. It is proposed that the drop exudation represents a kidney-like function of the extramatrical hyphae and a bridge to drier soil particles to initiate nutrient uptake by the hyphae. The ecological function of the different extramatrical structures of ho fungi are discussed. The ho cords or hyphae may translocate water only in the vessels or symplastically and not in the cell walls. The ho property may be essential among the S-selected (stress-tolerant) factors in these forest fungi. The transfer from water-repelling exploring structures into more hi exploiting structures in water contact with surrounding soil debris is, therefore, of great importance. The hi fungi did not form rhizomorph-like strands, in most cases, but an extending hyphal mycelium, representing foraging, exploring and exploiting structures at the same time. In the field, short strands may be found. On the hi fungi droplets were also produced but readily fused into a water sheath around the hypha. The hyphae thus tended to wick water via the cell wall.
Similar content being viewed by others
References
Agerer R (ed) (1987–1991) Color atlas of ectomycorrhizae. Einhorn, Schwäbisch Gmünd
Agerer R (1988) Studies on ectomycorrhizae. XVII. The ontogeny of the ectomycorrhizal rhizomorphs of Paxillus involutus and Thelephora terrestris (Basidiomycetes). Nova Hedwigia Kryptogamenkd 47:311–334
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD (1989) Molecular biology of the cell, 2nd edn. Garland, New York London
Ashford AE, Shepherd V, Orlovich D (1993) Phosphorus transfer and vacuoles in mycorrhizas. Abstracts, 9th North American Conference on Mycorrhizae, University of Guelph, Ontario, Canada, p 70
Bending GD (1992) Distribution and function of the ectomycorrhizal mycelium. Abstracts, 9th North American Conference on Mycorrhizae, University of Guelph, Ontario, Canada, p 143
Brownlee C, Duddridge JA, Malibari A, Read DJ (1983) The structure and function of mycelial systems of ectomycorrhizal roots with special reference to their role in forming inter-plant connections and providing pathways for assimilate and water transport. Plant Soil 71:433–443
Cairney JWG (1992) Translocation of solutes in ectomycorrhizal and saprophytic rhizomorphs. Mycol Res 96:135–141
Danell E, Fries N (1990) Methods for isolation of Cantharellus species, and the synthesis of mycorrhiza with Picea abies. Mycotaxon 38:141–148
Duchesne LC, Ellis B E, Peterson RL (1989) Disease suppression by the ectomycorrhizal fungus Paxillus involutus: contribution of oxalic acid. Can J Bot 67:2726–2730
Dütsch GA (1978) Submerged culture of Agaricus bisporus in a syntheic medium. Bull Br Mycol Soc 12:119
Finley RD, Read DJ (1986) The structure and function of the vegetative mycelium of ectomycorrhizal plants. I. Translocation of 14-C-labelled carbon between plants interconnected by a common mycelium. New Phytol 103:143–156
Fries N, Bardet M, Serck-Hansen K (1985) Growth of ectomycorrhizal fungi stimulated by lipids from a pine root exudate. Plant Soil 86:287–290
Grabski S, Feijter AW de, Schindler M (1993) Endoplasmic reticulum forms a dynamic continuum for lipid diffusion between contiguous soybean root cells. Plant Cell 5:25–38
Griffin D (1981) Fungal physiology. Wiley, New York
Jennings DH, Bravery AE (eds) (1991) Serpula lacrymans: fundamental biology and control strategies. Wiley, New York
Lehninger AL (1975) Biochemistry, 2nd edn. Worth, New York
Olsson S. Jennings DH (1991) Evidence for diffusion being the mechanism of translocation in the hyphae of three molds. Exp Mycol 15:302–309
Rayner ADM (1991) The challenge of the individualistic mycelium. Mycologia 83:48–71
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47:376–391
Stenström E (1991) The effects of flooding on the formation of ectomycorrhizae in Pinus sylvestris seedlings. Plant Soil 131:247–250
Straatsma G, Bruinsma J (1986) Carboxylated metabolic intermediates as nutritional factors in vegetative growth of the mycorrhizal mushroom Cantharellus cibarius Fr. J Plant Physiol 125:377–381
Tsao Y-H, Evans DF, Wennerström H (1993) Long-range attractive force between hydrophobic surfaces observed by atomic force microscopy. Science 262:547–550
Unestam T (1991) Water repellency, mat formation, and leafstimulated growth of some ectomycorrhizal fungi. Mycorrhiza 1:13–20
Unestam T, Stenström E (1989) A method for observing and manipulating roots and root-associated fungi on plants growing in nonsterile substrates. Scand J For Res 4:51–58
Wessels JH (1993) Wall growth, protein excretion and morphogenesis in fungi. Tansley review no 45. New Phytol 123:397–413
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Unestam, T., Sun, YP. Extramatrical structures of hydrophobic and hydrophilic ectomycorrhizal fungi. Mycorrhiza 5, 301–311 (1995). https://doi.org/10.1007/BF00207402
Issue Date:
DOI: https://doi.org/10.1007/BF00207402