Abstract
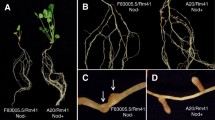
From a pool of Medicago truncatula mutants—obtained by gamma-irradiation or ethyl methanesulfonate mutagenesis—impaired in symbiosis with the N-fixing bacterium Sinorhizobium meliloti, new mutants are described and genetically analysed, and for already reported mutants, complementary data are given on their phenotypic and genetic analysis. Phenotypic data relate to nodulation and mycorrhizal phenotypes. Among the five new mutants, three were classified as [Nod+ Fix− Myc+] and the mutations were ascribed to two loci, Mtsym20 (TRV43, TRV54) and Mtsym21 (TRV49). For the two other new mutants, one was classified as [Nod−/+ Myc+] with a mutation ascribed to gene Mtsym15 (TRV48), and the other as [Nod− Myc-/+] with a mutation ascribed to gene Mtsym16 (TRV58). Genetic analysis of three previously described mutants has shown that [Nod−/+ Myc+] TR74 mutant can be ascribed to gene Mtsym14, and that [Nod−/+ Myc−/+] TR89 and TRV9 mutants are ascribed to gene Mtsym2 (dmi2). Using a detailed analysis of mycorrhizal phenotype, we have observed a delayed typical arbuscular mycorrhizal formation on some mutants that present thick lens-shaped appressoria. This phenotype was called [Myc−/+] and mutants TR25, TR26, TR89, TRV9, P1 and Y6 were reclassified as [Myc−/+]. Mutant P1 was reclassified as [Nod−/+] because of a late nodulation observed on roots of this mutant.
Similar content being viewed by others
References
Ané JM, Kiss GB, Riely BK, Penmetsa RV, Oldroyd GED, Ayax C, Lévy J, Debelle F, Baek JM, Kalo P, Rosenberg C, Roe BA, Long SR, Denarie J, Cook DR (2004) Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303:1364–1367
Barker DJ, Bianchi S, Blondon F, Datté Y, Duc G, Essad S, Flament P, Gallusci P, Grénier G, Guy P, Muel X, Tourneur J, Dénarié J, Huguet T (1990) Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium-legume symbiosis. Plant Mol Biol Rep 8:40–49
Calantzis C, Morandi D, Gianinazzi-Pearson V (2001) Cellular interactions between Glomus mosseae and a Myc−, Nod− mutant in Medicago truncatula. Symbiosis 30:97–108
Carroll BJ, McNeil DL, Gresshoff PM (1985) Isolation and properties of soybean (Glycine max (L.) Merr.) mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci USA 82:4162–4166
Catoira R, Galera C, deBilly F, Penmetsa RV, Journet EP, Maillet F, Rosenberg C, Cook D, Gough C, Dénarié J (2000) Four genes of Medicago truncatula controlling components of a nod factor transduction pathway. Plant Cell 12:1647–1665
Catoira R, Timmers ACJ, Maillet F, Galera C, Penmetsa RV, Cook D, Dénarié J, Gough C (2001) The HCL gene of Medicago truncatula controls Rhizobium-induced root hair curling. Development 128:1507–1518
Cook DR (1999) Medicago truncatula—a model in the making! Commentary. Curr Opin Plant Biol 2:301–304
Dénarié J, Cullimore J (1993) Lipo-oligosaccharide nodulation factors: signalling molecules mediating recognition and morphogenesis. Cell 74:951–954
Duc G (1995) Mutagenesis of faba bean (Vicia faba L.) and the identification of five different genes controlling no nodulation, ineffective nodulation or supernodulation. Euphytica 83:147–152
Duc G, Messager A (1989) Mutagenesis of pea (Pisum sativum L.) and the isolation of mutants for nodulation and nitrogen fixation. Plant Sci 60:207–213
Duc G, Trouvelot A, Gianinazzi-Pearson V, Gianinazzi S (1989) First report of non-mycorrhizal plant mutants (Myc−) obtained in pea (Pisum sativum L.) and faba bean (Vicia faba L.). Plant Sci 60:215–222
Egli MA, Griffith SM, Miller SS, Anderson MP, Vance CP (1989) Nitrogen assimilating enzyme activities and enzyme protein during development and senescence of effective and plant gene-controlled ineffective alfalfa nodules. Plant Physiol 91:898–904
Endre G, Kereszt A, Kevei Z, Mihacea S, Kalo P, Kiss GB (2002) A receptor kinase gene regulating symbiotic nodule development. Nature 417:962–966
Gao LL, Delp G, Smith SE (2001) Colonisation patterns in a mycorrhiza-defective mutant tomato vary with different arbuscular-mycorrhizal fungi. New Phytol 151:477–491
Gianinazzi-Pearson V, Gianinazzi S, Guillemin JP, Trouvelot A, Duc G (1991) Genetic and cellular analysis of resistance to vesicular arbuscular (VA) mycorrhizal fungi in pea mutants. In: Hennecke H, Verma DPS (eds) Advances in molecular genetics of plant-microbe interactions, vol 1. Kluwer, Dordrecht, pp 336–342
Gresshoff PM (1993) Molecular genetic analysis of nodulation genes in soybean. Plant Breed Rev 11:275–318
Lévy J, Bres C, Geurts R, Chalhoub B, Kulikova O, Duc G, Journet EP, Ane JM, Lauber E, Bisseling T, Denarie J, Rosenberg C, Debelle F (2004) A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303:1361–1364
Marsh JF, Schultze M (2001) Analysis of arbuscular mycorrhizas using symbiosis-defective plant mutants. New Phytol 150:525–532
Paruvangada VG, Davis TM (1999) A dominant host plant mutation conferring ineffective nodulation in chickpea-rhizobium symbiosis. J Hered 90:297–299
Penmetsa RV, Cook DR (2000) Production and characterisation of diverse developmental mutants of Medicago truncatula. Plant Physiol 123:1387–1397
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Sagan M, Gresshoff PM (1996) Developmental mapping of nodulation events in pea (Pisum sativum L.) using supernodulating plant genotypes and bacterial variability reveals both plant and Rhizobium control of nodulation regulation. Plant Sci 117:167–179
Sagan M, Morandi D, Tarenghi E, Duc G (1995) Selection of nodulation and mycorrhizal mutants in the model plant Medicago truncatula (Gaertn) after gamma-ray mutagenesis. Plant Sci 111:63–71
Sagan M, de Larembergue H, Morandi D (1998) Genetic analysis of symbiosis mutants in Medicago truncatula. In: Elmerich C, Kondorosi A, Newton WE (eds) Biological nitrogen fixation for the 21st century. Kluwer, Dordrecht, pp 317–318
Szcyglowski K, Shaws RS, Wopereis J, Copeland S, Hamburger D, Kasiborski B, Dazzo FB, de Bruin FJ (1998) Nodule organogenesis and symbiotic mutants of model legume Lotus japonicus. Mol Plant Microbe Interact 11:684–697
Verma DPS (1992) Signals in root nodule organogenesis and endocytosis of rhizobium. Plant Cell 4:373–382
Wais RJ, Galera C, Olroyd G, Catoira R, Penmetsa RV, Cook D, Gough C, Dénarié J, Long SL (2000) Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc Natl Acad Sci USA 97:13407–13412
Acknowledgements
We are grateful to Henri de Larembergue and Estelle Carteret for technical assistance, and to Richard Thompson for improving this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morandi, D., Prado, E., Sagan, M. et al. Characterisation of new symbiotic Medicago truncatula (Gaertn.) mutants, and phenotypic or genotypic complementary information on previously described mutants. Mycorrhiza 15, 283–289 (2005). https://doi.org/10.1007/s00572-004-0331-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-004-0331-4