Abstract
Although the recommended preoperative cessation period for sodium-glucose cotransporter 2 inhibitors (SGLT2is) changed in 2020 (from 24 h to 3–4 days preoperatively) to reduce the risk of SGLT2i-associated perioperative ketoacidosis (SAPKA), the validity of the new recommendation has not been verified. Using case reports, we assessed the new recommendation effectiveness and extrapolated precipitating factors for SAPKA. We searched electronic databases up to June 1, 2022 to assess SAPKA (blood pH < 7.3 and blood or urine ketone positivity within 30 days postoperatively in patients taking SGLT2i). We included 76 publications with 99 cases. The preoperative SGLT2i cessation duration was reported for 59 patients (59.6%). In all cases with available cessation periods, the SGLT2is were interrupted < 3 days preoperatively. No SAPKA cases with > 2-day preoperative cessation periods were found. Many case reports lack important information for estimating precipitating factors, including preoperative SGLT2i cessation period, body mass index, baseline hemoglobin A1c level, details of perioperative fluid management, and type of anesthesia. Our study suggested that preoperative SGLT2i cessation for at least 3 days could prevent SAPKA. Large prospective epidemiologic studies are needed to identify risk factors for SAPKA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sodium-glucose cotransporter 2 inhibitors (SGLT2is) are a relatively new class of anti-diabetic drugs. They lower blood glucose levels by promoting urinary glucose excretion via the inhibition of SGLT2, expressed in the proximal tubules. Their beneficial effects include reduced hemoglobin A1c (HbA1c) levels, weight loss, and a reduction in blood pressure [1]. Furthermore, recent large clinical trials have demonstrated that they have cardio- and reno-protective effects [2], prompting the US Food and Drug Administration (FDA) to approve them for treating heart failure and chronic kidney diseases, even in patients without diabetes [3, 4]. Available data demonstrate a good tolerability profile for SGLT2i with minor side effects such as genital mycotic infections, urinary tract infections, and increased urination [5]. However, they cause ketoacidosis, a rare but potentially life-threatening side effect. Over the last few years, many patients treated with SGLT2i have reportedly developed severe ketoacidosis [6]. Previous studies have suggested that surgery is a precipitating factor for developing SGLT2i-associated perioperative ketoacidosis (SAPKA), which is attributed to perioperative fasting and metabolic changes caused by surgical stress [7, 8]. In March 2020, the US FDA approved a label change to promote SGLT2i interruption before elective surgery, recommending a longer period (at least 3 days for canagliflozin, dapagliflozin, and empagliflozin, and 4 days for ertugliflozin) than that recommended previously (24 h preoperatively) to reduce the risk of ketoacidosis [9]. However, it is unclear whether this label change is effective in preventing SAPKA because no studies have validated the new recommendation. Furthermore, risk factors for SAPKA have not been completely elucidated given its low incidence. The primary objective of this study was to evaluate the validity of the updated recommendation concerning preoperative cessation of SGLT2is by summarizing the preoperative withholding period in a series of case reports. The secondary objective was to identify risk factors for SAPKA by summarizing the available data. As no clinical trials aiming to determine the potential adverse effects of this class of drug have been published, we conducted this study as a systematic review of case reports.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [10]. The protocol was published as a preprint before study initiation (https://www.medrxiv.org/content/10.1101/2022.05.22.22275348v1).
Eligibility criteria
We included case reports or series describing patients receiving SGLT2i who developed ketoacidosis (defined as blood pH < 7.3 and blood or urine ketone positivity) pre-, intra-, or postoperatively up to 30 days. Letters, reviews, and conference abstracts were also included. Patients who were newly prescribed SGLT2i postoperatively and those who did not fulfill the ketoacidosis criteria were excluded. Cases in which the pH value was unclear, but the authors stated “acidosis” were included.
Search strategy and study selection
Two independent researchers (H.S. and S.I.) searched electronic databases, including PubMed, EMBASE, and Web of Science, up to June 1, 2022, with no language restrictions for identifying perioperative ketoacidosis associated with SGLT2is. The full search strategy is summarized in Supplementary Table S1. The reference lists of all identified studies and those of previous meta-analyses on similar topics were checked. Two reviewers (H.S. and S.I.) independently screened the obtained references’ titles and abstracts and collected full-text articles if potentially relevant. Disagreements were resolved through discussion or consultation with a third author (K.Y.).
Data extraction
Fourteen reviewers (H.S., S.I., H.W., M.O., A.M., H.N., K.K., T.Y., H.H., S.H., M.S., A.I., M.H., and T.O.) working in seven teams independently extracted data on age, sex, body mass index (BMI), the purpose of SGLT2i prescription, comorbidities, diabetes type, SGLT2i type, anti-diabetic and other medications, surgery and anesthesia type, data on withholding agents preoperatively, nature of presentation as ketoacidosis, biochemical parameters (including serum glucose, HbA1c, pH, serum bicarbonate [HCO3], partial pressure of arterial carbon dioxide [PaCO2], anion gap [AG], plasma and urine ketones, and glycosuria), management details, complications, and outcomes.
Assessment of methodological quality of the cases
The methodological quality of the cases was assessed using a previously published method (Supplementary Table S2) [11]. Each case was assessed in five domains, for which a binary (Yes/No) response was generated. The quality of the report was graded from 0 (low) to 5 (high). Disagreements were resolved through discussion or consultation with a third author (K.Y.).
Statistical analysis
Since this was a systematic review of case reports, descriptive epidemiology was performed on predetermined items for each case. Standard descriptive statistics were performed on those reported numerically, and pooled analysis was not performed. For continuous variables with a normal distribution, the mean (standard deviation) was reported. For non-normally distributed variables, the median (range) was reported. Data were analyzed using the PRISM 6 software (GraphPad Software, Inc., CA).
Results
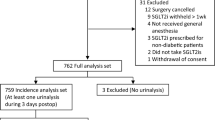
This study was conducted according to the pre-published protocol. The search strategy identified 2934 citations, among which 252 were assessed in full-text; 76 publications with 99 cases were included in the review (Fig. 1) [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87]. Eighty-one cases included in this study were reported in published papers, and the remaining 18 were reported in meeting abstracts. Each case’s summary is presented in Supplementary Table S3. The reports’ methodological quality was graded as 5 in 64 cases, 4 in 13 cases, 3 in 14 cases, and 2 in eight cases. Most cases were reported in the US (n = 32), followed by Australia (n = 15), Canada (n = 9), UK (n = 6), and Japan (n = 6). The number of reported cases has increased since the first case was reported in 2015 (Fig. 2).
Backgrounds of reported cases
The patients’ mean age was 57 years (Supplementary Fig. S1). Most patients with SAPKA were female (male, 37; female, 55; “undefined”; 7 cases). BMI was reported in only 25 (25.3%) cases, with a median (range) of 38 (16–49) kg/m2. SGLT2i was prescribed for treating diabetes in 96 (97.0%) patients, among which 82 had type 2 diabetes, three had type 1 diabetes, and no information was provided regarding the type of diabetes in 11 patients. Dapagliflozin was prescribed for heart failure treatment in a non-diabetes patient [72]. Comorbidities included hypertension (n = 31), coronary artery disease (n = 14), cerebrovascular disease (n = 5), and renal dysfunction (n = 3). Information regarding comorbidities in 40 patients was unavailable. The anesthesia method (n = 91) and medications other than anti-diabetic drugs (n = 81) were not reported in most cases. In 37 (37.4%) cases, baseline HbA1c values were reported, with a mean (standard deviation) of 8.5% (1.5%) (Supplementary Fig. S2). Details regarding preoperative diabetes medication in 75 cases are described in Supplementary Table S4. Postoperative glycosuria was reported in 23 cases; however, the urine glucose levels of the remaining 76 cases were not described. Bariatric surgery was the most common type of surgery (n = 24) in the reported cases, followed by coronary artery bypass grafting (CABG) (n = 18) and cholecystectomy (n = 5) (Supplementary Table S5). Four of the 20 patients undergoing CABG underwent emergency surgery, and 12 underwent elective surgery. Overall, 19 patients underwent emergency surgery and 60 underwent elective surgery. The urgency of the surgery was unknown in the remaining 20 patients. Anesthesia methods were not described in 91 cases. Although general anesthesia tends to be administered in most cases, this was only specified in eight cases. The preoperative fasting period was described in 20 cases (1 day; 11 cases, 3 days; one case, and 5–16 h; eight cases). Perioperative fluid management details have not been described in most cases (Supplementary Table S6).
Management of SGLT2i
The SGLT2is prescribed in the reported cases were canagliflozin, empagliflozin, dapagliflozin, and “undefined” in 42, 32, 16, and 9 cases, respectively. The treatment duration before the event was not reported in 72 cases. In the remaining 27 patients, the medication duration varied between 1 month and 6 years. The duration of preoperative SGLT2i cessation was reported for 58 patients (58.6%) (Supplemental Table S7). SGLT2i use was ceased 1 day (n = 33), 2 days (n = 9), 18 h (n = 1) or 42 h (n = 1) preoperatively or not ceased at all (n = 14). SGLT2i was omitted perioperatively in one case; however, the duration of preoperative cessation was unclear. There were no preoperative SGLT2i cessation descriptions in the other 40 (40.4%) cases. In all reportedly available cases, SGLT2i administration was stopped in < 3 days before surgery. Among the 19 patients who underwent emergency surgery, four, five, four, and six patients took SGLT2is on the day of surgery, 1 day preoperatively, 2 days preoperatively, or “undefined,” respectively. Postoperatively, SGLT2i was resumed or continued in 15 cases, discontinued in 26, and not reported in 58 cases.
Characteristics of SAPKA
Table 1 shows a summary of the characteristics of ketoacidosis. The time to diagnose ketoacidosis was reported for 86 (86.9%) patients. Among them, ketoacidosis was diagnosed within 3 days in 57 (66.3%) patients, 10 days in 74 (86.0%), and 14 days in 77 (89.5%) postoperatively. Ketoacidosis was diagnosed intraoperatively in two cases. Ketoacidosis was identified based on laboratory data in 29 cases, whereas 60 presented with symptoms such as nausea, vomiting, respiratory symptoms, and tachycardia. The basis for identifying ketoacidosis was unclear in 10 patients. Regarding blood samples, β-hydroxybutyrate was measured in 45 cases and ketones in 23. The pH value during the diagnosis of ketoacidosis was reported for 93 (93.9%) cases; conversely, the evidence of acidosis was unclear in the remaining 6. HCO3, PaCO2, and AG values were reported in 87 (87.9%), 54 (54.5%), and 70 (70.7%) cases, respectively. Details of these values are presented in Table 1 and Supplementary Fig. S3. Ketone positivity was detected in urine samples (22 cases), blood samples (43 cases), and both (32 cases). The ketone measurement method was unclear in two cases.
Blood glucose levels at diagnosis were reported in 93 cases (93.9%); however, the specific value was unclear in 13 cases because it was reported as a range. In the remaining 80 patients, the median [range] blood glucose level during diagnosis was 179 [52–498] mg/dL (Supplementary Fig. S4). In eight cases (10%), the blood glucose level was normal (≤ 125 mg/dL). Sixty-nine (86.2%) patients were hyperglycemic (> 125 mg/dL); however, most patients (59/69, 85.5%) had mild hyperglycemia (≤ 250 mg/dL). Hypoglycemia (blood glucose level, 52 mg/dL) was detected in one non-diabetes patient who received dapagliflozin for heart failure treatment. The most frequently cited precipitating factor were fasting or reduced oral intake (n = 27), followed by surgery or surgical stress (n = 24) and inadequate insulin dose (n = 4).
Treatment and outcome
Treatment details for ketoacidosis, including fluid, glucose, insulin, and bicarbonate administration, were described in 90 cases. In three cases, the “DKA protocol” was applied to treat ketoacidosis. Most patients (79/99 cases, 79.8%) recovered from ketoacidosis, 40 required ICU admission, seven required mechanical ventilation, and three required dialysis. One patient died from stroke after Moyamoya revascularization surgery. Outcomes were not reported in 19 cases.
Discussion
In this systematic review of case reports on SAPKA, no SAPKA cases with > 2-day preoperative cessation periods were found, suggesting that a longer cessation period may reduce the risk of developing SAPKA. Although the withholding period was not reported in approximately 40% of cases, this finding is important, as no studies have validated the updated recommendation of preoperative SGLT2i cessation. However, the optimal withholding period remains uncertain. Some patients presented with persistent postoperative glycosuria lasting 3 days or longer despite SGLT2i withdrawal [22, 32, 45, 73], suggesting that SGLT2i has residual efficacy. In this study, approximately 60% of SAPKA occurred within 3 days postoperatively. However, 27.2% developed later than 3 days up to 28 days postoperatively. The specific period of time after discontinuing the medication in which disease onset occurs is unclear. Since in most cases postoperative urine glucose data were not provided (72/99), future studies should present the cessation duration of SGLT2is as well as information regarding perioperative urine glucose to estimate the optimal preoperative withholding period.
As we expected, the number of SAPKA case reports has increased steeply, even after the SGLT2i package insert was revised in 2020. This is likely due to the rapid increase in SGLT2i use as well as increased awareness of SAPKA. Because SAPKA may be easily overlooked, there is an urgent need to elucidate its risk factors. We have made some discoveries in this regard. First, we present evidence that emergency surgery is a risk factor for SAPKA. Here, emergency surgery accounted for a quarter of SAPKA cases. Notably, among 19 patients who underwent emergency surgery, at least nine patients did not take SGLT2i on the day of surgery, indicating that taking SGLT2i on the day of surgery was not the only cause of the SAPKA. Other factors, such as decreased oral intake, dehydration, and infection in these populations, may increase the risk of SAPKA. Second, SAPKA was predominantly observed in female patients. Sex differences in the frequency of SGLT2i-associated ketoacidosis have not been reported to the best of our knowledge. However, a previous study of ketoacidosis associated with canagliflozin suggested that patients prone to increase ketone bodies tended to have advanced type 2 diabetes mellitus (DM) [88]. Thus, the results of the current study, in which SAPKA was predominantly observed in women, might be affected by the baseline severity of DM. Unfortunately, baseline HbA1c values were reported only in 37.4% of cases. Further studies are needed to determine the effect of the severity of DM and sex on the incidence of SAPKA.
In terms of surgery type, bariatric surgery was the most common, which is consistent with the previous study results [11]. Although the effect of obesity on the incidence of SAPKA was unclear, as BMI was reported only in 25.3% of cases, considering that obese patients are prone to ketosis with increased lipolysis, and bariatric surgery is quite common worldwide, the results of our study clearly indicated that bariatric surgery is one of the most important risk factors for SAPKA. Except for bariatric surgery, more invasive surgeries and background infections pose a higher risk of SAPKA.
As mentioned above, most case reports lack important information, other information should be reported. First is a detailed description of perioperative fluid management and glucose and insulin administration. Although there is limited evidence for recommending optimal fluid management for diabetes patients undergoing surgery [89], inappropriate fluid and glucose management can increase the risk of SAPKA [90,91,92]. Second, the anesthetic method has not been specified in most cases. Perioperative stress response and blood glucose level can be affected by the anesthesia type [93, 94]. Therefore, future studies should include these data.
Some SGLT2is have been indicated for the treatment of heart failure and renal failure in 2020 and 2021, respectively. We found only one case of SAPKA in patients prescribed SGLT2i for non-diabetic indication. In this case, dapagliflozin was prescribed for the treatment of heart failure and continued till the day of surgery. The patient developed SAPKA secondary to postoperative hypoglycemia despite the administration of 1% glucose during surgery. Previous studies have suggested that SGLT2is do not increase the risk of hypoglycemia when compared with placebos outside the perioperative setting [95, 96]. However, this case suggested that SAPKA can occur in non-diabetes patients treated with SGLT2i. Unlike diabetic indication, blood glucose may not be routinely measured in non-diabetes patients, and the optimal withholding duration in non-diabetes patients treated with SGLT2is for heart failure is unknown, as discontinuing the drug may be deleterious to heart failure management. Clinicians should be aware of the risk of SGLT-2-associated hypoglycemia and ketoacidosis in perioperative settings in non-diabetes patients taking these drugs for heart failure.
In most cases, ketoacidosis was not immediately diagnosed, but most patients recovered without complications, indicating that prompt diagnosis and appropriate treatment are correlated with good/improved prognoses.
Limitations
First, to evaluate preoperative SGLT2i-cessation recommendations and the risk of developing ketoacidosis, it would be necessary to include SGLT2i-treated patients who did not develop ketoacidosis. The exclusion of these patients would make it difficult to completely evaluate SGLT2i-cessation guidelines and would cause a likelihood of biases, such as reporting and selection biases. Second, the duration of preoperative cessation of SGLT2is was reported in only 59 (< 60%) patients. Although all of these patients stopped their SGLT2i < 3 days before surgery, it is difficult to conclusively assess the validity of the updated guidelines. We are currently conducting a multicenter observational study to estimate the incidence and precipitating factors of SAPKA [97]. In the study, the duration of preoperative SGLT2i cessation varied among patients and facilities because the recommendation was changed after the study was initiated, which could help determine the validity of the new recommendation. Third, we found only one case report describing SAPKA in a non-diabetes patient. However, this does not mean that the non-diabetic indication of the SGLT2i does not occur, as their prescriptions are expected to increase rapidly given their recognition as an exciting new treatment option for patients with kidney disease and heart failure [98]. Fourth, we excluded cases in which blood pH was > 7.30, even if blood or urine ketone bodies were elevated. This may exclude cases in which ketosis developed due to SGLT2is. However, ketosis is sometimes physiological and non-fatal. Although a previous systematic review included some ketosis cases, we described a robust SAPKA feature by including only cases that fulfilled the criteria for ketoacidosis.
In conclusion, this study suggests that SGLT2i cessation at least 3 days preoperatively can effectively prevent SAPKA. The steep increase in SAPKA case reports indicates that SAPKA is no longer a rare complication among diabetes patients on SGLT2i medications. However, this study could not identify risk factors for SAPKA, highlighting the need for large prospective epidemiologic studies to identify risk factors. Moreover, high-level vigilance, including postoperative ketone screening, may be recommended for high-risk populations.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66:255–70.
Caruso I, Giorgino F. SGLT-2 inhibitors as cardio-renal protective agents. Metabolism. 2022;127: 154937.
US Food and Drug Administration. FDA News Release FDA approves new treatment for a type of heart failure. 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure. Accessed 25 Jun 2022.
US Food and Drug Administration. FDA News Release FDA approves treatment for chronic kidney disease. 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease. Accessed 25 Jun 2022.
Halimi S, Vergès B. Adverse effects and safety of SGLT-2 inhibitors. Diabetes Metab. 2014;40:S28-34.
Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA adverse event reporting system. Diabetologia. 2017;60:1385–9.
Ata F, Yousaf Z, Khan AA, Razok A, Akram J, Ali EAH, Abdalhadi A, Ibrahim DA, Al Mohanadi DH, Danjuma MI. SGLT-2 inhibitors associated euglycemic and hyperglycemic DKA in a multicentric cohort. Sci Rep. 2021;11:10293.
Meyer EJ, Gabb G, Jesudason D. SGLT2 inhibitor-associated euglycemic diabetic ketoacidosis: a South Australian clinical case series and Australian spontaneous adverse event notifications. Diabetes Care. 2018;41:e47–9.
US Food and Drug Administration. FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious#:~:text=Safety%20and%20Availability-,FDA%20revises%20labels%20of%20SGLT2%20inhibitors%20for%20diabetes%20to%20include,and%20serious%20urinary%20tract%20infections&text=To%20lessen%20the%20risk%20of,information%20for%20SGLT2%20inhibitor%20medicines. Accessed 25 Jun 2022.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Thiruvenkatarajan V, Meyer EJ, Nanjappa N, Van Wijk RM, Jesudason D. Perioperative diabetic ketoacidosis associated with sodium-glucose co-transporter-2 inhibitors: a systematic review. Br J Anaesth. 2019;123:27–36.
Abu-Amer N, Dinour D, Mini S, Beckerman P. An unusual case of metabolic acidosis: clinical case education. Isr Med Assoc J. 2019;21:766–8.
Aggarwal A, Jain A, Sachdeva S, Kulairi ZI. Prolonged glucosuria with sodium-glucose cotransporter-2 (SGLT2) inhibitors: a case report and review of literature. Cureus. 2020;12: e11554.
Alabdaljabar MS, Abdullah KM, Almasood A, Ali SS, Ashmeg A. Euglycemic diabetic ketoacidosis in a sedated patient after coronary artery bypass grafting: a case report and literature review. Case Rep Med. 2021;2021:2086520.
Amianda EA, Gavigan TS, Talishinskiy T, Ewing DR, Schmidt HJ. Two cases of euglycemic diabetic ketoacidosis after bariatric surgery associated with sodium-glucose cotransporter-2 inhibitor use. Obes Surg. 2021;31:3848–50.
Andalib A, Elbahrawy A, Alshlwi S, Alkhamis A, Hu W, Demyttenaere S, Aggarwal R, Court O. Diabetic ketoacidosis following bariatric surgery in patients with type 2 diabetes. Diabetes Care. 2016;39:e121–2.
Banakh I, Kung R, Gupta S, Matthiesson K, Tiruvoipati R. Euglycemic diabetic ketoacidosis in association with dapagliflozin use after gastric sleeve surgery in a patient with type II diabetes mellitus. Clin Case Rep. 2019;7:1087–90.
Bobart SA, Gleason B, Martinez N, Norris K, Williams SF. Euglycemic ketoacidosis caused by sodium-glucose cotransporter 2 inhibitors: a case report. Ann Intern Med. 2016;165:530–2.
Bonanni FB, Fei P, Fitzpatrick LL. Normoglycemic ketoacidosis in a postoperative gastric bypass patient taking canagliflozin. Surg Obes Relat Dis. 2016;12:e11–2.
Bteich F, Daher G, Kapoor A, Charbek E, Kamel G. Post-surgical euglycemic diabetic ketoacidosis in a patient on empagliflozin in the intensive care unit. Cureus. 2019;11: e4496.
Chacko B, Whitley M, Beckmann U, Murray K, Rowley M. Postoperative euglycaemic diabetic ketoacidosis associated with sodium-glucose cotransporter-2 inhibitors (gliflozins): a report of two cases and review of the literature. Anaesth Intensive Care. 2018;46:215–9.
Chong ZM, Stirling A, Sandeep T. Sodium-glucose cotransporter 2 (SGLT-2): cause of prolonged euglycaemic ketoacidosis and ketonaemia following general anaesthesia? Diabet Med. 2018;35:100.
Chow YY, Worsley R, Topliss DJ. Lessons from the bedside: ketoacidosis and SGLT2 inhibitors. Med J Aust. 2016;205:191–2.
Curanaj FM, Seley J. Euglycemic DKA in a patient with type 2 diabetes on an SGLT2 inhibitor: is it a zebra? Endocr Pract. 2020;26:142–55.
Darwish AM. Metabolic acidosis in postsurgical patient on canagliflozin and metformin: a case report. A A Pract. 2019;12:221–2.
Di Palma R, Vanderpuye A, Satara J, Ceronie B, Trenfield S. A case of empagliflozin-induced euglycaemic diabetic ketoacidosis post-off pump coronary artery bypass surgery. Diabet Med. 2020;37:78.
Dizon S, Keely EJ, Malcolm J, Arnaout A. Insights into the recognition and management of SGLT2-inhibitor-associated ketoacidosis: it’s not just euglycemic diabetic ketoacidosis. Can J Diabetes. 2017;41:499–503.
Ejaz S, Patel A, Oiknine R. Recognizing low endogenous insulin production as a precipitating factor of DKA in type 2 diabetic patients taking SGLT2 inhibitors: a case report. Endocr Pract. 2016;22:98.
Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38:1680–6.
Evans LC, Salahuddin S. Just sweet enough? A case of euglycaemic ketoacidosis “incidentally” found in a post surgical patient. Diabet Med. 2018;35:105.
Gelaye A, Haidar A, Kassab C, Kazmi S, Sinha P. Severe ketoacidosis associated with canagliflozin (Invokana): a safety concern. Case Rep Crit Care. 2016;2016:1656182.
Ghofrani H, Wong C, Smogoizewski M. Severe ketoacidosis after bariatric surgery in a patient treated with canagliflozin. Kidney Week. 2015;26:342A.
Gomez-Sanchez CM, Wu BX, Gotts JE, Chang RW. Euglycemic diabetic ketoacidosis following major vascular surgery is a new item on the differential for postoperative acidosis. J Vasc Surg Cases Innov Tech. 2021;7:778–80.
Gonzalez ME, Velez JCQ. A case of perioperative euglycemic ketoacidosis and concomitant non-anion gap metabolic acidosis and AKI associated with canagliflozin. J Am Soc Nephrol. 2017;28:937–8.
Goto S, Ishikawa JY, Idei M, Iwabuchi M, Namekawa M, Nomura T. Life-threatening complications related to delayed diagnosis of euglycemic diabetic ketoacidosis associated with sodium-glucose cotransporter-2 inhibitors: a report of 2 cases. Am J Case Rep. 2021;22: e929773.
Hamill CF, Hunter SJ. A case of euglycaemic DKA in a perioperative patient taking an SGLT2 inhibitor for type 2 diabetes. Ir J Med Sci. 2017;186:S367–8.
Hawkins A, Jackson R, White H, Vardesh D. SGLT2-inhibitor induced euglycemic ketoacidosis in acute surgical patients. J Case Rep Images Surg. 2017;3:41–6.
Hoffman C, Green M, Megafu O. Sodium-glucose linked transporter 2 inhibitor-associated perioperative euglycaemic diabetic ketoacidosis: a case for a perioperative guideline. Anaesth Intensive Care. 2017;45:758.
Horikoshi R, Kitamura T, Miyaji K. Ketoacidosis related to sodium glucose cotransporter 2 inhibitors after emergency coronary surgery. Interact Cardiovasc Thorac Surg. 2019;29:323–4.
Howard J, Wiegley N, Madan N. Severe euglycemic diabetic ketoacidosis secondary to sodium-glucose cotransporter 2 (SGLT2) inhibitor: a diagnostic challenge. J Am Soc Nephrol. 2019;30:1198.
Iqbal QZ, Mishiyev D, Niazi MR, Zia Z, Sattar SBA, Jahanghir A, Quyyumi S. SGLT-2 Inhibitors-a culprit of diabetic ketoacidosis postbariatric surgery. Case Rep Crit Care. 2020;2020:8817829.
Isaacs M, Tonks KT, Greenfield JR. Euglycaemic diabetic ketoacidosis in patients using sodium-glucose co-transporter 2 inhibitors. Intern Med J. 2017;47:701–4.
Jhaveri U, Vardesh D. Sodium-glucose cotransporter-2 inhibitors and euglycaemic diabetic ketoacidosis in the perioperative period: case report. Cureus. 2019;11: e5455.
Kameda Y, Kato M, Inoue B, Yamazaki S. Euglycemic diabetic ketoacidosis caused by a sodium-glucose cotransporter 2 inhibitor after coronary artery bypass grafting. J Am Coll Cardiol. 2019;73:2423.
Kapila V, Topf J. Sodium-glucose co-transporter 2 inhibitor-associated euglycemic diabetic ketoacidosis after bariatric surgery: a case and literature review. Cureus. 2021;13: e17093.
Kitahara C, Morita S, Kishimoto S, Matsuno S, Uraki S, Takeshima K, Furukawa Y, Inaba H, Iwakura H, Ariyasu H, Furuta H, Nishi M, Akamizu T. Early detection of euglycemic ketoacidosis during thoracic surgery associated with empagliflozin in a patient with type 2 diabetes: a case report. J Diabetes Investig. 2021;12:664–7.
Klosko R, Rozycki A, Lester J. Diagnosis and management of SGLT-2 inhibitor-induced euglycemic DKA after cardiac surgery. Crit Care Med. 2021;49:211.
Kuchay MS, Mishra SK, Mehta Y. Empagliflozin induced euglycemic diabetic ketoacidosis in a patient undergoing coronary artery bypass graft despite discontinuation of the drug 48 hours prior to the surgery. Diabetes Metab Syndr. 2021;15:909–11.
Lane S, Mohammed S, Paskar D, Goffi A. ‘Euglycemic’ diabetic ketoacidosis in the perioperative period: Why gliflozins may not be so sweet after all! Am J Respir Crit Care Med. 2017;195.
Lau A, Bruce S, Wang E, Ree R, Rondi K, Chau A. Perioperative implications of sodium-glucose cotransporter-2 inhibitors: a case series of euglycemic diabetic ketoacidosis in three patients after cardiac surgery. Can J Anaesth. 2018;65:188–93.
Lee D, Khan O, Reynolds M, Mahler K. The sugar-free diabetic! Ketoacidosis in disguise. Anaesthesia. 2021;76:13.
Li J, Dizon S, Arnaout A. SGLT-2 inhibitors & ketacidosis in bariatric patients: a cases series. Endocr Rev. 2016;37.
Lindsay PJ, Gibson LE, Bittner EA, Berg S, Chang MG. Sodium-glucose cotransporter-2 (SGLT2) inhibitor-induced euglycemic diabetic ketoacidosis complicating the perioperative management of a patient with type 2 diabetes mellitus (T2DM) and Fournier’s gangrene: a case report. Int J Surg Case Rep. 2020;77:463–6.
Luo X, Ji R, Lu W, Zhu H, Li L, Hu J. Dapagliflozin-associated euglycemic diabetic ketoacidosis in a patient who underwent surgery for pancreatic carcinoma: a case report. Front Surg. 2022;9: 769041.
Mackintosh C, Tewari A, Siegel J, Wang RD, Freeman W. Postoperative euglycemic diabetic ketoacidosis and encephalopathy related to SGLT-2 inhibitors: a case report and discussion of diabetes treatment and “sweet pee encephalopathy” in perioperative hospital management. Neurohospitalist. 2020;10:51–4.
Malicek D, Wagner S, Kaiser S, Khandoga A, Meininger D. Severe condition of shock with combined lactic and ketoacidosis in a patient after left-sided pancreas resection under permanent therapy with metformin and dapagliflozin. Anasthesiol Intensivmed. 2020;61:472.
McCabe DE, Strollo BP, Fuhrman GM. Euglycemic diabetic ketoacidosis in the surgical patient. Am Surg. 2020. https://doi.org/10.1177/0003134820956352.
McGuiness M, Paschke K, Foreman B. Euglycemic DKA from an SGLT2 inhibitor in a heart transplant patient. J Am Coll Cardiol. 2021;77:2268.
Mendonça FM, Silva MM, Chaves V, Souto S, Freitas P, Carvalho D. Diabetic ketoacidosis after bariatric surgery: a case report. Rev Port Endocrinol Diabetes Metab. 2021;15:178–81.
Menhem M, Kunstadt R. Euglycemic diabetic ketoacidosis in type 2 diabetes mellitus following sleeve gastrectomy. Diabetes. 2017;66:A347.
Milne L, Bryant C. Severe euglycaemic ketoacidosis with SGLT2 inhibitor use in the perioperative period. Anaesthesia. 2019;74:12.
Misaghian-Xanthos N, Shariff AI, Mekala K, Fearrington LR, Setji TL, Aloi JA, Buse JB. Sodium-glucose cotransporter 2 inhibitors and diabetic ketoacidosis: a case series from three academic institutions. Diabetes Care. 2017;40:e65–6.
Mossler J, Hwang S. A saccharin case of diabetic ketoacidosis. J Hosp Med. 2018;13.
Noubleau AMA, Narang DK. Development of euglycemic diabetic ketoacidosis in postbariatric surgery patients with type 2 diabetes mellitus on canaglifozin. Endocr Rev. 2018;39.
Osafehinti DA, Okoli OJ, Karam JG. A case of SGLT2 inhibitor-associated euglycemic diabetic ketoacidosis following coronary artery bypass surgery. AACE Clin Case Rep. 2021;7:20–2.
Pang J, Malik RQ, Ho SY, Saha S, Hussain N. Sodium glucose co-transporter 2 (SGLT2) Inhibitor: How well do we know the side effect profile in ITU? Should current perioperative diabetes guideline be challenged? J Intensive Care Soc. 2018;19:122–6.
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1687–93.
Pontes JPJ, de Melo CS, Arantes FBB, de Souza Ramos JTG, Módolo NSP, Navarro ELLH. Perioperative euglycemic diabetic ketoacidosis following use of SGLT-2 inhibitors after cardiac surgery. J Clin Anesth. 2021;71:110201.
Rafey MF, Butt A, Coffey B, Reddington L, Devitt A, Lappin D, Finucane FM. Prolonged acidosis is a feature of SGLT2i-induced euglycaemic diabetic ketoacidosis. Endocrinol Diabetes Metab Case Rep. 2019;2019:19–0087.
Sampani E, Sarafidis P, Dimitriadis C, Kasimatis E, Daikidou D, Bantis K, Papanikolaou A, Papagianni A. Severe euglycemic diabetic ketoacidosis of multifactorial etiology in a type 2 diabetic patient treated with empagliflozin: case report and literature review. BMC Nephrol. 2020;21:276.
Seger CD, Xing H, Wang L, Shin JS. Intraoperative diagnosis of sodium-glucose cotransporter 2 inhibitor-associated euglycemic diabetic ketoacidosis: a case report. A A Pract. 2021;15: e01380.
Seki H, Watanabe H, Yorozu T. Postoperative ketoacidosis with hypoglycemia in a nondiabetic patient taking dapagliflozin for heart failure: a case report. A A Pract. 2022;16: e01570.
Shah M, Pathrose E, Bhagwat NM, Chandy D. ‘The Bitter Truth of Sugar’-euglycemic diabetic ketoacidosis due to sodium-glucose cotransporter-2 inhibitors: a case series. Indian J Crit Care Med. 2022;26:123–6.
Tansey DJ, Pazderska A, Healy ML. Please don’t sugarcoat it: an avoidable case of euglycaemic DKA in the setting of a SGLT-2 Inhibitor. Ir J Med Sci. 2019;188:S248–9.
Theodore D, Pongracz B, Titus B. Euglycemic ketoacidosis in a postoperative deformity correction spine surgery patient: a case report. J Emerg Crit Care Med. 2018;2:83.
Tsai MK, Chen GL, Chuang TJ. Dapagliflozin-induced postoperative euglycemic diabetic ketoacidosis: two cases. J Intern Med Taiwan. 2019;30:47–50.
Ullah S, Khan N, Zeb H, Tahir H. Metabolic ketoacidosis with normal blood glucose: a rare complication of sodium-glucose cotransporter 2 inhibitors. SAGE Open Med Case Rep. 2016;4:2050313X16675259.
Vadi S, Lad V, Kapoor D. Perioperative implication of sodium-glucose cotransporter-2 inhibitor in a patient following major surgery. Indian J Crit Care Med. 2021;25:958–9.
van Niekerk C, Wallace J, Takata M, Yu R. Euglycaemic diabetic ketoacidosis in bariatric surgery patients with type 2 diabetes taking canagliflozin. BMJ Case Rep. 2018;2018:bcr2017221527.
Wang KM, Isom RT. SGLT2 inhibitor-induced euglycemic diabetic ketoacidosis: a case report. Kidney Med. 2020;2:218–21.
Wang Q, Wu K, Luo X, Dong X, Liu W, Tang Z, Zhang B. Dapagliflozin-associated euglycemic diabetic ketoacidosis presenting with severe abdominal pain mimicking acute peritonitis. Cureus. 2022;14: e22229.
Wang R, Kave B, McIlroy E, Kyi M, Colman PG, Fourlanos S. Metabolic outcomes in patients with diabetes mellitus administered SGLT2 inhibitors immediately before emergency or elective surgery: Single centre experience and recommendations. Br J Anaesth. 2021;127:e5–7.
Wohlrab P, Kainz M, Schiferer A, Zapletal B, Tschernko E. Euglycemic diabetic ketoacidosis after cardiac surgery in a patient treated with empagliflozin for type 2 diabetes mellitus. J Cardiothorac Vasc Anesth. 2022;36:2066–9.
Wong YC, Liu KL, Lee CL. Postoperative extremity gangrene in a patient with type 2 diabetes taking SGLT2 inhibitors: a case report. Medicine. 2021;100: e25590.
Wood T, Pang AJ, Hallet J, Greig P. Euglycaemic ketoacidosis in a postoperative Whipple patient using canaglifozin. BMJ Case Rep. 2016;2016:bcr2016216607.
Yared KE, Mancini GJ. Euglycemic diabetic ketoacidosis associated with use of SGLT2 inhibitor after laparoscopic Roux-en-Y gastric bypass. Am Surg. 2021;87:1997–9.
Zhang L, Tamilia M. Euglycemic diabetic ketoacidosis associated with the use of a sodium-glucose cotransporter-2 inhibitor. CMAJ. 2018;190:E766–8.
Polidori D, Iijima H, Goda M, Maruyama N, Inagaki N, Crawford PA. Intra- and inter-subject variability for increases in serum ketone bodies in patients with type 2 diabetes treated with the sodium glucose co-transporter 2 inhibitor canagliflozin. Diabetes Obes Metab. 2018;20:1321–6.
Membership of the Working Party, Barker P, Creasey PE, Dhatariya K, Levy N, Lipp A, Nathanson MH, Penfold N, Watson B, Woodcock T. Peri-operative management of the surgical patient with diabetes 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia. 2015;70:1427–40.
Galway U, Chahar P, Schmidt MT, Araujo-Duran JA, Shivakumar J, Turan A, Ruetzler K. Perioperative challenges in management of diabetic patients undergoing non-cardiac surgery. World J Diabetes. 2021;12:1255–66.
Dhatariya K, Levy N, Kilvert A, Watson B, Cousins D, Flanagan D, Hilton L, Jairam C, Leyden K, Lipp A, Lobo D, Sinclair-Hammersley M, Rayman G, Joint British Diabetes Societies. NHS Diabetes guideline for the perioperative management of the adult patient with diabetes. Diabet Med. 2012;29:420–33.
Jackson MJ, Patvardhan C, Wallace F, Martin A, Yusuff H, Briggs G, Malik RA, Nwrag Peri-op Diabetes Audit Group. Perioperative management of diabetes in elective patients: a region-wide audit. Br J Anaesth. 2016;116:501–6.
Zhang Y, Su T, Li R, Yan Q, Zhang W, Xu G. Effect of multimodal analgesia on perioperative insulin resistance in patients with colon cancer. Indian J Cancer. 2021;58:349–54.
Li X, Wang J, Chen K, Li Y, Wang H, Mu Y, Chen Y. Effect of different types of anesthesia on intraoperative blood glucose of diabetic patients: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2017;96: e6451.
Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–74.
Pelletier R, Ng K, Alkabbani W, Labib Y, Mourad N, Gamble JM. Adverse events associated with sodium glucose co-transporter 2 inhibitors: an overview of quantitative systematic reviews. Ther Adv Drug Saf. 2021;12:2042098621989134.
Seki H, Kuratani N, Shiga T, Iwasaki Y, Karita K, Yasuda K, Yorozu T. Multicentre prospective observational study of sodium-glucose cotransporter-2 inhibitor-associated postoperative ketoacidosis: the SAPKA study protocol. BMJ Open. 2021;11: e049592.
van der Aart-van der Beek AB, de Boer RA, Heerspink HJL. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat Rev Nephrol. 2022;18:294–306.
Funding
Japan Society for the Promotion of Science (Grant no. 21K06676).
Author information
Authors and Affiliations
Contributions
HS serves as the contact person of this review. Conceiving the review: HS. Designing the review: HS, SI, NK, TS, KY. Coordinating the review: HS, SI. Data collection for the review: HS, SI, HW, MO, AM, HN, KK, TY, HH, SH, MS, AN, MH, TO. Data management for the review: HS. Analysis of data: HS, NK, TS. Interpretation of data: HS, SI, HW, MO, AM, HN, KK, TY, HH, SH, MS, AN, MH, TO. Writing the review: HS, SI, NK. Providing general advice on the review: TS, KY. Writing the protocol: HS, NK.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Yasuda reports receiving grants from Ono Pharmaceutical and Mitsubishi Tanabe Pharma Corporation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Seki, H., Ideno, S., Shiga, T. et al. Sodium-glucose cotransporter 2 inhibitor-associated perioperative ketoacidosis: a systematic review of case reports. J Anesth 37, 465–473 (2023). https://doi.org/10.1007/s00540-023-03174-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-023-03174-8