Abstract
Background
The bispectral index (BIS) value during general anesthesia with the newly developed anesthetic remimazolam is reported to be relatively high; however, the reason for this and the appropriate indicator for assessing the sedation level during remimazolam anesthesia have not been determined. In this study, the level of sedation during general anesthesia with remimazolam was evaluated using several different indicators.
Methods
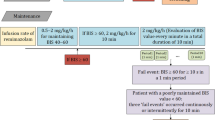
Thirty patients who underwent breast surgery under general anesthesia with remimazolam were included. BIS®, Sedline® and the pupil resting diameters were measured simultaneously. The intraoperative dose of remimazolam was adjusted to obtain a BIS in the range of 40–60; if a BIS < 60 could not be achieved, the intraoperative dose was increased up to the maximal dose of 2 mg/kg/h.
Results
The mean intraoperative BIS and patient state index (PSI) in all patients was 50.6 ± 9.1 and 43.0 ± 11.8, respectively. Five patients showed a mean intraoperative BIS > 60 and eight patients showed mean intraoperative PSI > 50. The mean intraoperative spectral edge frequency (SEF) of BIS® or Sedline® was 15.3 ± 2.5 Hz or 10.6 ± 3.0 Hz, each. The mean intraoperative resting pupil diameter was 1.7 ± 0.2 mm. There were no patients with awareness during anesthesia.
Conclusions
Processed electroencephalograms (BIS and PSI), and SEF of BIS® were relatively high during anesthesia with remimazolam, but SEF of Sedline® or pupillary diameter could be a supportive indicator to confirm sedation level during remimazolam anesthesia.



Similar content being viewed by others
References
Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, Wiard RP, Feldman PL, Collins H, Waszczak BL, Tilbrook GS. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107(1):60–6.
Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012;115(2):284–96.
Upton RN, Somogyi AA, Martinez AM, Colvill J, Grant C. Pharmacokinetics and pharmacodynamics of the short-acting sedative CNS 7056 in sheep. Br J Anaesth. 2010;105(6):798–809.
Pastis NJ, Yarmus LB, Schippers F, Ostroff R, Chen A, Akulian J, Wahidi M, Shojaee S, Tanner NT, Callahan SP, Feldman G, Lorch DG Jr, Ndukwu I, Pritchett MA, Silvestri GA, Investigators P. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155(1):137–46.
Chen S, Wang J, Xu X, Huang Y, Xue S, Wu A, Jin X, Wang Q, Lyu J, Wang S, Li Y, Yu Y, Ai D, Luo A, Min S, Li L, Zou X, Liu J, Lv P, Chai X, Sun X, Zhao Z, Zhang J. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res. 2020;12(8):4594–603.
Rex DK, Bhandari R, Desta T, DeMicco MP, Schaeffer C, Etzkorn K, Barish CF, Pruitt R, Cash BD, Quirk D, Tiongco F, Sullivan S, Bernstein D. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–37.
Borkett KM, Riff DS, Schwartz HI, Winkle PJ, Pambianco DJ, Lees JP, Wilhelm-Ogunbiyi K. A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120(4):771–80.
Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543–53.
Schuttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–51.
Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34(4):491–501.
Zhou J, Leonowens C, Ivaturi VD, Lohmer LL, Curd L, Ossig J, Schippers F, Petersen KU, Stoehr T, Schmith V. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020;66:109899.
Lohmer LL, Schippers F, Petersen KU, Stoehr T, Schmith VD. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020;60(4):505–14.
Larson MD, Sessler DI, McGuire J, Hynson JM. Isoflurane, but not mild hypothermia, depresses the human pupillary light reflex. Anesthesiology. 1991;75(1):62–7.
Leslie K, Sessler DI, Smith WD, Larson MD, Ozaki M, Blanchard D, Crankshaw DP. Prediction of movement during propofol/nitrous oxide anesthesia. Performance of concentration, electroencephalographic, pupillary, and hemodynamic indicators. Anesthesiology. 1996;84(1):52–63.
Shirozu K, Setoguchi H, Tokuda K, Karashima Y, Ikeda M, Kubo M, Nakamura K, Hoka S. The effects of anesthetic agents on pupillary function during general anesthesia using the automated infrared quantitative pupillometer. J Clin Monit Comput. 2017;31(2):291–6.
Shirozu K, Murayama K, Yamaura K. Pupillary response as assessment of effective seizure induction by electroconvulsive therapy. J Vis Exp. 2019. https://doi.org/10.3791/59488.
Minto CF, Schnider TW, Shafer SL. Pharmacokinetics and pharmacodynamics of remifentanil. II. Model application. Anesthesiology. 1997;86(1):24–33.
Upton RN, Martinez AM, Grant C. Comparison of the sedative properties of CNS 7056, midazolam, and propofol in sheep. Br J Anaesth. 2009;103(6):848–57.
Eisenried A, Schuttler J, Lerch M, Ihmsen H, Jeleazcov C. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part II. Pharmacodynamics of electroencephalogram effects. Anesthesiology. 2020;132(4):652–66.
Miyake W, Oda Y, Ikeda Y, Hagihira S, Iwaki H, Asada A. Electroencephalographic response following midazolam-induced general anesthesia: relationship to plasma and effect-site midazolam concentrations. J Anesth. 2010;24(3):386–93.
Greenblatt DJ, Ehrenberg BL, Gunderman J, Locniskar A, Scavone JM, Harmatz JS, Shader RI. Pharmacokinetic and electroencephalographic study of intravenous diazepam, midazolam, and placebo. Clin Pharmacol Ther. 1989;45(4):356–65.
Sleigh J, Pullon RM, Vlisides PE, Warnaby CE. Electroencephalographic slow wave dynamics and loss of behavioural responsiveness induced by ketamine in human volunteers. Br J Anaesth. 2019;123(5):592–600.
Koitabashi T, Johansen JW, Sebel PS. Remifentanil dose/electroencephalogram bispectral response during combined propofol/regional anesthesia. Anesth Analg. 2002;94(6):1530–3 (Table of contents).
Doi M, Gajraj RJ, Mantzaridis H, Kenny GN. Relationship between calculated blood concentration of propofol and electrophysiological variables during emergence from anaesthesia: comparison of bispectral index, spectral edge frequency, median frequency and auditory evoked potential index. Br J Anaesth. 1997;78(2):180–4.
Veselis RA, Reinsel RA, Feshchenko VA, Wronski M. The comparative amnestic effects of midazolam, propofol, thiopental, and fentanyl at equisedative concentrations. Anesthesiology. 1997;87(4):749–64.
Bulach R, Myles PS, Russnak M. Double-blind randomized controlled trial to determine extent of amnesia with midazolam given immediately before general anaesthesia. Br J Anaesth. 2005;94(3):300–5.
Acknowledgements
We would like to express our deepest appreciation to Mrs. Yoko Kondo, who works at the Kyushu University Hospital, for her assistance in this study. We also thank Editage® for English editing service.
Author information
Authors and Affiliations
Contributions
KS: methodology, formal analysis, writing—original draft, writing—review and editing. KN, ST, KI, ST, KN: data curation, writing—review. MH, KY: writing—review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
No external funding and no competing interests declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Shirozu, K., Nobukuni, K., Tsumura, S. et al. Neurological sedative indicators during general anesthesia with remimazolam. J Anesth 36, 194–200 (2022). https://doi.org/10.1007/s00540-021-03030-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-021-03030-7