Abstract
Background and aims
The use of conventional oral sulfate tablets (OSTs) has gained popularity; nonetheless, they may be not only inconvenient to swallow but also difficult to dissolve. A novel mini-OST has recently been developed to enhance compliance with conventional OST use. This study aimed to compare the efficacy, tolerability, and safety between mini-OST and conventional OST.
Methods
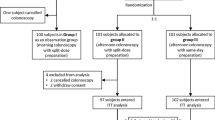
This was a prospective, randomized, investigator-blinded, multicenter, and non-inferior phase 3 trial conducted between September 2022 and December 2022. The efficacy, safety, and tolerability were compared between mini-OST and conventional OST.
Results
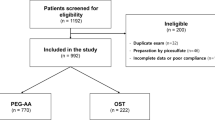
Exactly 83 patients were evaluated based on a full analysis set (FAS), whereas 82 patients were evaluated as a per-protocol set (PPS). With respect to the efficacy of preparation, successful and high-quality preparation was excellent in the mini-OST and conventional OST groups on both FAS and PPS analyses, without significant differences between the two groups. On the FAS analysis, the satisfaction and tolerability scores were high in both groups, without significant differences. The first bowel movement after taking the investigational product occurred 30 min earlier in the mini-OST group than in the OST group. Mild, moderate, and severe adverse events (AEs) were comparable between the two groups; however, any AEs were more common in the mini-OST group than in the conventional OST group overall.
Conclusions
Compared with conventional OST, the novel mini-OST preparation showed similar efficacy, tolerability, and safety, including mild eight solicited AEs and moderate-to-severe AEs.
Clinical trial registration NCT05670470.



Similar content being viewed by others
References
Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–105.
Loberg M, Kalager M, Holme O, et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 2014;371:799–807.
Cohen LB, Sanyal SM, Von Althann C, et al. Clinical trial: 2-L polyethylene glycol-based lavage solutions for colonoscopy preparation - a randomized, single-blind study of two formulations. Aliment Pharmacol Ther. 2010;32:637–44.
Hassan C, East J, Radaelli F, et al. Bowel preparation for colonoscopy: European society of gastrointestinal endoscopy (ESGE) Guideline—update 2019. Endoscopy. 2019;51:775–94.
DeMicco MP, Clayton LB, Pilot J, et al. Novel 1 L polyethylene glycol-based bowel preparation NER1006 for overall and right-sided colon cleansing: a randomized controlled phase 3 trial versus trisulfate. Gastrointest Endosc. 2018;87(677–87): e3.
Anastassopoulos K, Farraye FA, Knight T, et al. A comparative study of treatment-emergent adverse events following use of common bowel preparations among a colonoscopy screening population: results from a post-marketing observational study. Dig Dis Sci. 2016;61:2993–3006.
Di Palma JA, Bhandari R, Cleveland MV, et al. A safety and efficacy comparison of a new sulfate-based tablet bowel preparation versus a PEG and ascorbate comparator in adult subjects undergoing colonoscopy. Am J Gastroenterol. 2021;116:319–28.
Kim JH, Park YE, Kim TO, et al. Comparison of the efficacy and safety between oral sulfate tablet and polyethylene glycol for bowel preparation before colonoscopy according to age. Medicine (Baltimore). 2022;101: e29884.
Lee SE, Oh DJ, Nam JH, et al. Taking oral sulfate tablets with simethicone for bowel preparation leads to higher adenoma detection rate than polyethylene glycol: a propensity score analysis. Dig Dis Sci. 2023;68:867–76.
Yang HJ, Park DI, Park SK, et al. Novel sulfate tablet PBK-1701TC versus oral sulfate solution for colon cleansing: A randomized phase 3 trial. J Gastroenterol Hepatol. 2020;35:29–36.
Eduardo VMB, Wisam Z. Oral sulfate tablet bowel preparation is associated with erosive gastritis. Am J Gastroenterol. 2021;2021:S263.
Halphen M, Heresbach D, Gruss HJ, et al. Validation of the Harefield Cleansing Scale: a tool for the evaluation of bowel cleansing quality in both research and clinical practice. Gastrointest Endosc. 2013;78:121–31.
Hatoum HT, Lin SJ, Joseph RE, et al. Validation of a patient satisfaction scale in patients undergoing bowel preparation prior to colonoscopy. Patient. 2016;9:27–34.
Bisschops R, Manning J, Clayton LB, et al. Colon cleansing efficacy and safety with 1 L NER1006 versus 2 L polyethylene glycol + ascorbate: a randomized phase 3 trial. Endoscopy. 2019;51:60–72.
Rex DK, McGowan J, Cleveland M, et al. A randomized, controlled trial of oral sulfate solution plus polyethylene glycol as a bowel preparation for colonoscopy. Gastrointest Endosc. 2014;80:482–91.
Radaelli F, Paggi S, Hassan C, et al. Split-dose preparation for colonoscopy increases adenoma detection rate: a randomised controlled trial in an organised screening programme. Gut. 2017;66:270–7.
Bitoun A, Ponchon T, Barthet M, et al. Results of a prospective randomised multicentre controlled trial comparing a new 2-L ascorbic acid plus polyethylene glycol and electrolyte solution vs. sodium phosphate solution in patients undergoing elective colonoscopy. Aliment Pharmacol Ther. 2006;24:1631–42.
Arya V, Gupta KA, Valluri A, et al. Rapid colonoscopy preparation using bolus lukewarm saline combined with sequential posture changes: a randomized controlled trial. Dig Dis Sci. 2013;58:2156–66.
Matro R, Shnitser A, Spodik M, et al. Efficacy of morning-only compared with split-dose polyethylene glycol electrolyte solution for afternoon colonoscopy: a randomized controlled single-blind study. Am J Gastroenterol. 2010;105:1954–61.
Rex DK, Di Palma JA, Rodriguez R, et al. A randomized clinical study comparing reduced-volume oral sulfate solution with standard 4-liter sulfate-free electrolyte lavage solution as preparation for colonoscopy. Gastrointest Endosc. 2010;72:328–36.
Song JH, Bae JH, Yim JY. Efficacy of oral sulfate tablets for bowel preparation and adenoma detection rate. J Gastroenterol Hepatol. 2023;38:410–5.
Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72–90.
Baker FA, Mari A, Nafrin S, et al. Predictors and colonoscopy outcomes of inadequate bowel cleansing: a 10-year experience in 28,725 patients. Ann Gastroenterol. 2019;32:457–62.
Tongprasert S, Sobhonslidsuk A, Rattanasiri S. Improving quality of colonoscopy by adding simethicone to sodium phosphate bowel preparation. World J Gastroenterol. 2009;15:3032–7.
Rachh PR, Rahane RD. A review on fast dissolving tablet. J Drug Deliv Ther. 2018;8:50–5.
Di Palma JA, Rodriguez R, McGowan J, et al. A randomized clinical study evaluating the safety and efficacy of a new, reduced-volume, oral sulfate colon-cleansing preparation for colonoscopy. Am J Gastroenterol. 2009;104:2275–84.
Markowitz GS, Stokes MB, Radhakrishnan J, et al. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389–96.
Acknowledgements
The authors thank Korean Association for the Study of Intestinal Diseases (KASID) and the clinical trial department of Taejoon Pharmaceuticals for their expertise.
Funding
This study was a sponsor-initiated study funded by Taejoon Pharm Co., Seoul, Korea.
Author information
Authors and Affiliations
Contributions
SRJ was involved in collection and interpretation of data, and drafting of the manuscript. SKP and DHY contributed to collection and interpretation of data. JMC collected and interpreted data, and was responsible for the integrity of the data and accuracy of the data analysis, study design, and supervision. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no conflicts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jeon, S.R., Park, SK., Yang, DH. et al. Comparison of a novel mini-oral sulfate tablet and the conventional oral sulfate tablet in bowel preparation for colonoscopy: a prospective, randomized, investigator-blinded, multicenter, non-inferior, phase 3 trial. J Gastroenterol 58, 1114–1123 (2023). https://doi.org/10.1007/s00535-023-02023-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-023-02023-5