Abstract
Many clinical trials have been conducted for inflammatory bowel disease (IBD), so various clinical indices (CIs) and endoscopic indices (EIs) have also been evaluated. However, recently, with the progress of IBD management, review of established indices from previous studies, and establishment of new indices, the landscape of the use of indices in clinical trials have changed. We investigated the number and frequency of the indices adapted in recent clinical trials for ulcerative colitis (CI and EI) and Crohn’s disease (CI, EI, index related to magnetic resonance imaging, index for evaluating patient-reported outcomes, and health-related quality of life). Based on the results, we selected representative indices and further reviewed their content and characteristics. Moreover, various definitions, including clinical and endoscopic response or remission, have been described by means of representative indices in clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) is treated based on the activity and extent of the disease. Evaluations using the clinical index (CI) and endoscopic index (EI) are useful in determining the appropriate treatment, predicting prognosis, and evaluating or monitoring disease activity after treatment [1, 2]. Generally, clear treatment evaluation is important while performing clinical trials for IBD. However, treatment goals have diversified into clinical, endoscopic, pathological, and psychological goals recently [1,2,3]. Additionally, the indices used in recent clinical trials have changed due to the review of previously established indices, establishment of new indices, and diversification of treatment goals, such as mucosal healing (MH) and pathological healing (PH). In response to this background, the Selecting Therapeutic Target in IBD (STRIDE) program was established in 2013 by the International Organization for the Study of IBD. An achievement of this program, STRIDE-I was presented in 2015 as a recommended treat-to-target (T2T) approach based on the evidence and consensus of experts [4]. In 2020, a more updated STRIDE-II was presented [5]. Practicing T2T is important for long-term treatment strategies for IBD [4,5,6], and STRIDE-II describes the importance of achieving treatment goals, which are suitable for early, middle, and long-term treatments [5]. The disease activity index based on clinical symptoms; biomarkers, such as C-reactive protein (CRP) or fecal calprotectin (FC), endoscopic index, and histological index, are widely used to monitor disease activity. In this review, we first clarified the indices adopted frequently in clinical trials for IBD, and derived representative indices for ulcerative colitis (UC) and Crohn’s disease (CD). We then outlined how clinical and endoscopic remissions were defined in these clinical trials provided that each index has problems with its characteristics and definitions. The purpose of this review was to optimize the method of evaluating disease activity in IBD and help select and define appropriate indicators when planning clinical trials.
Ulcerative colitis
UC is a refractory, chronic, IBD involving the large intestine. Activity index requires accuracy and validity for selecting its treatment and evaluating its efficacy on a patient post treatment [3, 7,8,9]. The establishment of effective treatments, such as biologics, ensured the improvement of therapeutic goals, including endoscopic or histological remission, and the improvement of symptoms that had been defined as conventional goals. Therefore, as the evaluation items or the content required by an index used continue to change, the index adopted in clinical trials also changes. For example, the placebo effect is not suppressed by the evaluation of the CI comprising only subjective symptoms as their subjective evaluations have limitations [1, 7, 10, 11]. Therefore, most recent clinical trials for UC have adopted biomarker levels or endoscopic evaluation as the main parameters for objective evaluation. Clinical trials for UC frequently adopt the Mayo Score, which includes the CI-independent endoscopic score or the EI as a sub-score item. Considering these, to clarify the circumstances of adopted indices in recent clinical trials performed for UC, we conducted a comprehensive literature search through PubMed with a survey period spanning from January 2009 to December 2017. In 2010, Hirai et al. carefully read 100 papers with “ulcerative colitis” and “clinical trial” in the search string from 2001 to 2006 and reported the frequency of CIs and EIs used [12]. Thereafter, an additional survey was conducted using the same method during the search period of 1999–2008 as a framework of the Ministry of Health, Labour and Welfare of Japan. In creating this review, we followed the research method of Hirai et al. and investigated the indices used in studies in 2009–2017. To clarify the change of the index used over time, the research results were divided into three categories for each survey period: 1999–2008, 2009–2012, and 2013–2017.
With “ulcerative colitis” and “clinical trial” included in the search string, 296 articles regarded as evaluated with CI or EI were extracted and read through carefully. Figure 1 shows the flow diagram of the study selection process and exclusion criteria (e.g., insufficient descriptions, abstract only, general remarks, letters, and studies not targeting humans). Tables 1 and 2 show the frequency of CI and EI use based on these research results.
Flow diagram of the study selection process for UC. We performed an electronic search using PubMed. The survey period was from January 2009 to December 2017. The search string we used included “ulcerative colitis” and “clinical trial” as key words. We carefully read the studies that were extracted, and 296 studies were considered to have been evaluated by an index
The indices revealed to be used most frequently in previous studies, considered to be highly valid, and appeared to be frequently applicable in future were selected, and their respective development reasons, evaluation items, characteristics, and various definitions were reviewed. Additionally, the histopathological, pediatric UC, and postoperative ileal pouchitis indices were described in sections independent from the results of the research.
Results of electronic research for UC
In terms of most recent (2013–2017) CIs for adults, Mayo Score (49.5%), Rachmilewitz Index (also known as Clinical Activity Index, CAI) (16.5%), Sutherland Index (also known as Disease Activity Index, DAI) (13.7%), Simple Clinical Colitis Activity Index (SCCAI) (11.5%), and Lichtiger Index (2.2%) have been most adopted.
In terms of most recent (2013 to 2017) EIs, Mayo Endoscopic Sub-score (MES) (69.0%) and Sutherland Endoscopic Sub-score (19.8%), regarded as a sister index for MES, accounted for an overwhelmingly large percentage. They were followed by the Baron index (5.6%), Rachmilewitz endoscopic sub-score (4.8%), and Ulcerative Colitis Endoscopic Index of Severity (UCEIS) (4.8%).
The survey results clarified that the usage rate of Mayo score (including partial Mayo score) has been increasing in recent CIs. It also clarified that the usage rate of Mayo score (including Mayo endoscopic sub-score) has been increasing for recent EIs. In addition, the recently developed Ulcerative Colitis Endoscopic Index of Severity (UCEIS) was used for the evaluations in some recent studies.
Among CIs, the Mayo, Sutherland, Rachmilewitz, Simple Clinical Colitis Activity, and Lichtiger indices were described. For the EIs, the Sutherland endoscopic sub-score is regarded as nearly similar to MES, so only MES was selected. Along with UCEIS, whose validity has been evaluated, MES was described based on the results of adoption frequency. Additionally, the histopathological, pediatric UC, and postoperative ileal pouchitis indices were described in sections independent from the results of the research.
Clinical Index for UC
Mayo Score (Supplementary Table 1)
Schroeder et al. developed this index to evaluate the efficacy and safety of oral mesalazine [13]. Eighty-seven patients with mild to moderate active UC were administered mesalazine and placebo for six weeks, and evaluated in a randomized, double-blind, controlled trial. In the original article, the efficacy was evaluated by comparing the proportions of complete response (CR), partial response (PR), and no response in each administration group with those in the placebo group. The Mayo Score has since then been the most adapted index in recent clinical trials [5]. However, an index that is an independently modified Mayo Score is also frequently used. Since then, the Mayo Score has been frequently used as a partial Mayo Score (p-MS) or as a MES, combining one or multiple items of the sub-score. Although the Mayo Score is simple and frequently used, its validity has yet to be examined sufficiently, and its definitions of CR and MH have remained inconsistent. The MES is described independently in the section on EI.
Sutherland Index (Supplementary Table 2)
Sutherland et al. developed this index to easily evaluate the efficacy and safety of mesalazine enemas [14]. Endoscopic mucosal findings, which are quoted from the Baron Index, are applied to evaluate the activity. It is possible not only to evaluate the index as a whole, but also evaluate each evaluation item independently. Although it is composed of almost the same items as the Mayo Score, each evaluation item, except for those related to endoscopic findings, is evaluated based on the condition of a patient after 1 day, which is different from the 3-day period of the Mayo Score.
Rachmilewitz Index (Supplementary Table 3)
Rachmilewitz et al. developed this index to compare the efficacy and safety of mesalazine and sulfasalazine [15]. CAI and EI were proposed separately. CAI is evaluated based on the condition of a patient 1 week before further evaluation. EI scores each of the four characteristic findings of UC during its active period and uses their sum as the score. There is a discrepancy between the remission rates of CAI and EI, and CAI and EI do not necessarily correlate. Additionally, CAI has been validated, but EI has not.
Simple Clinical Colitis Activity Index (Supplementary Table 4)
Walmsley et al. developed this index to evaluate disease activity easily [16]. Based on the Powell-Tuck Index, bowel frequency (night) was included in the nighttime symptoms evaluated. In the original article, its evaluation items are described simply enough for non-specialists or even patients themselves to perform during initial evaluations at the outpatient department. Additionally, its high correlation with the scoring system of the Seo Index, which is a well-validated index including three objective contents (hemoglobin, erythrocyte segmentation ratio, and albumin), has been confirmed [16, 17].
Lichtiger Index (Supplementary Table 5)
Lichtiger et al. developed this index to evaluate the efficacy and safety of continuous intravenous cyclosporine therapy [18, 19]. The purpose of this index is to evaluate severe patients, and since the evaluation period for them is short, it focuses on short-term changes in symptoms. Therefore, it requires a condition in which the evaluation of efficacy is continuous for 2 consecutive days. Additionally, the reliability of this index is suggested by the fact that there was no difference in the evaluation of two or more physicians and that the improvement rate under blinding and after key opening were similar.
Endoscopic Index for UC
Mayo endoscopic sub-score
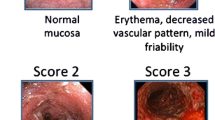
Among the evaluation items included in the Mayo Score, only items related to mucosal findings of the endoscopy were used independently as EI, which is called MES. Although it is just a simple, four-grade evaluation, it still has the following problems: the score may not change much before and after treatment and does not reflect partial or minor changes in vascular visibility.
Issues of evaluation of mucosal healing using MES
While the MES is simple and frequently used, its validity has yet to be sufficiently examined, and its definitions of CR and MH have remained inconsistent. A frequently used definition of MH is “MES = 0” or “MES = 1” [5, 20, 21]. However, there is a difference in the relapse rate between “MES = 0” and “MES = “1 [20,21,22,23,24,25]. Therefore, the achievement of MES 0 is recommended for better outcomes in the recent literature composed of voting results by IBD experts [5]. Additionally, the MES scores appear to vary depending on the evaluation site or physician.
Ulcerative Colitis Endoscopic Index of Severity (Supplementary Table 6)
Regarding EIs used before this index was established, differences in evaluation between physicians were pointed out, and none of them were validated. Travis et al. pointed out these problems, judged various endoscopic findings in UC reported by multiple physicians, verified their validity sufficiently, and identified three findings that are optimal for endoscopic activity evaluation: “Vascular pattern”, “Bleeding”, and “Erosions and ulcers”. A highly objective index was then developed in which these three findings were evaluated and scored separately at the site with the strongest findings prior to calculating for the total score [26, 27]. This index makes it possible to easily evaluate changes in each finding and to evaluate severity more objectively compared to the grading scale represented by MES. It also has the advantage of having a wide score range, 0–8 points, making the degree of improvement before and after treatment easier to evaluate objectively.
Issues of evaluation of mucosal healing using UCEIS
Although “endoscopic remission (ER)” or “MH” is not defined in the original article, there is a high consensus that it refers to “UCEIS = 0” or “UCEIS ≤ 1” [5, 21]. However, knowledge about the long-term prognosis and surgical rate of UC based on UCEIS is limited at present and needs further investigations [28,29,30].
Reproducibility between MES and UCEIS
A good correlation between MES and UCEIS was suggested (κ = 0.713, p < 0.001) [32]. In addition, when the weighted kappa index in MES was 0.8 (good), 0.52 (acceptable), and 0.49 (acceptable) in the evaluation of the same case by three endoscopists, the intra-class correlation coefficient of UCEIS was 0.92 (95% CI 0.83–0.96), indicating a correlation between MES and UCEIS [33]. On the other hand, since the degree of ulceration (shallow or deep) was not mentioned in the MES, it may lead to a discrepancy between MES and UCEIS [28].
Histopathological index for UC
The evaluation of UC activity has been based primarily on clinical symptoms and endoscopic findings [1]. However, the importance of histological improvement has recently been highlighted based on various histopathological indices [31, 34]. Conversely, the correlations between ER and histopathological remission (HR) and inter-observer reliability in the evaluation of histopathological inflammation are relatively weak [35, 36]. Therefore, the clinical utility of STRIDE-II on histological improvement is limited; in fact, it was deemed low by the Delphi method, positioning this problem as a gap that needs to be addressed by future investigations [5]. Currently, the achievement of HR is rarely set as the primary endpoint of UC treatment, but it is recommended that histopathological evaluation be set as a secondary endpoint [8]. To better illustrate this, this section describes the Matts, Riley, and Geboes scores.
Matts Classification (Supplementary Table 7)
Matts et al. developed this index for endoscopic and histopathological evaluation of UC in a review of rectal biopsy procedures and reported a simple and safe method [37]. Histological grades of the Matts classification are classified into five grades (1–5) based on histopathological findings (cellular infiltration, crypt abscesses, erosion, and ulcer).
Riley Score (Supplementary Table 8)
Riley et al. developed this index to evaluate relapse based on microscopic rectal inflammation [38]. It is a grading scale that evaluates six histopathological findings on a 4-point scale with the average of the evaluations made by the two pathologists considered as the final score. “Inflammatory cell infiltrate”, “crypt abscesses”, “mucin depletion”, and “breaches in the surface epithelium” showed a high correlation with relapse. It is important to note that this index is different from the grading systems developed by Riley et al. in 1988 [39]. Although a clear definition of remission is not shown in the original article, definitions, such as “Riley Score = 1” [40] and “Riley Score change ≥ Δ1” [40, 41], are applied as “remission” and “improvement”, respectively.
Geboes Score (Supplementary Table 9)
Indices for histopathological evaluation focusing on neutrophil infiltration have been proposed, but their validity has yet to be verified. Therefore, Geboes et al. developed this index to achieve clear validity [42]. The overall evaluation concordance rate was 65%, which was considered a “good or acceptable quality”. The main advantage of this index is that each evaluation item is evaluated independently. Unlike conventional indices, evaluation of activity is performed not only by one item, such as degree of neutrophil infiltration, but also by other evaluation items. Although the definition of remission is not clearly stated in the original article, patients with “Geboes Score ≤ 1.0” [43] and “Geboes Score < 2.0” [44] are considered in “remission”.
Index for Pediatric UC
Evaluating pediatric UC patients using the results of laboratory findings or imaging findings, including endoscopy, has proven to be more difficult than evaluating adult UC patients. Additionally, some indices that have been developed independently focus only on subjective symptoms. The earlier onset of UC is characterized by a higher tendency to become severe and develop into total colitis types [45, 46]. Unlike in adults, a higher dose of medication is recommended, pointing out the growing trend of dependence on steroids and immuno-modulators [47]. This section describes the Pediatric UC Activity Index (PUCAI), which is an index for pediatric UC that is regarded as important in terms of treatment selection.
Pediatric Ulcerative Colitis Activity Index (Supplementary Table 10)
Turner et al. developed this index to evaluate disease activity in pediatric UC cases with a noninvasive and accurate reflection [48]. Item reduction and instrument formatting were carried out using the Delphi technique, and the items were weighted using Physician’s Global Assessment (PGA). Good correlation was confirmed with PGA, Total Colonoscopy Activity Index and Mayo Score. Additionally, its inter-observer reliability (ICC > 0.87) and test–retest reliability (ICC = 0.94) were also good. Subsequent studies reported that PUCAI on days 3 and 5 of steroid therapy was useful in assessing steroid responsiveness and in deciding to move on to the next treatment [49, 50]. In STRIDE-II, this index is recommended as a CI for pediatric UC [5].
Index for postoperative ileal pouchitis
Although the surgery rate of UC has decreased due to advances in medical treatment, surgery is still needed for intractable cases with severe bleeding, perforation, addictive megacolon, and patients with colitis-associated dysplasia or cancer [51,52,53,54,55,56,57]. Postoperative complications such as ileal pouchitis are observed with a certain probability, and in some cases, it is difficult to control [56]. Objective evaluation of ileal pouchitis is therefore very important in determining the most appropriate management strategies and evaluating subsequent treatments. This section describes the Pouchitis Disease Activity Index (PDAI), which is an index of ileal pouchitis.
Pouchitis Disease Activity Index (Supplementary Table 11)
Sandborn et al. developed the PDAI to evaluate ileal pouchitis after ileal pouch-anal anastomosis, and compared it with previously reported indices for ileal pouchitis (Pouchitis Triad, Histopathology Index) [57]. PDAI showed significantly higher scores in patients who had clinical symptoms of ileal pouchitis than in other patients. The definition of pouchitis activity was not referred to in the original article.
Crohn’s disease
CD presents with complications, such as stenosis, fistula, and abscess, and approximately 50% of cases will undergo surgery within 10 years of diagnosis [54, 58]. Additionally, its risks for postoperative relapse, mainly at the anastomotic site, and complicating to colorectal cancer have been reported [54, 58, 59]. In such disabilities, the progression of disease behavior over time remains to be a problem. In recent years, various effective treatments, including biologics, have become generalized, and treatment targets such as “MH” and “deep remission” have become widespread. In addition, as with UC, the concept of a T2T approach that makes use of biomarker normalization to achieve the targeted ER has been presented [1, 2, 4, 5, 60]. However, there remains no consensus on the clear definitions of clinical and endoscopic responsiveness and remission, which are the goals of monitoring [60, 61]. In STRIDE-II, treatment targets are set for each period, and strategies that are more in line with actual clinical practice are recommended [5]. In future CD treatment, monitoring with biomarkers and endoscopy will be important, but it seems important to first clarify the circumstances of more recent clinical trials related to CD that made them adopt specific indices. In the same manner the UC indices were analyzed, these CD indices were investigated through a literature search.
We performed an electronic research using PubMed with a survey period spanning from January 2009 to December 2017. “Crohn’s disease” and “clinical trial” were included in the search string, and 405 articles that had been regarded as evaluated with CI, EI, index using magnetic resonance imaging (MRI), and index using patient-reported outcomes (PROs) or health-related quality of life (HR-QoL) were extracted and read through carefully. Figure 2 shows the flow diagram of the study selection process, including the exclusion criteria used. Tables 3, 4, 5, and 6 show the frequency of use of CI, EI, index related to MRI, and index related to PROs or HR-QoL based on these research results. The indices that were revealed to be used most frequently in previous studies, considered to be highly valid, and appeared to be frequently applicable in future, were selected, and their development reasons, evaluation items, characteristics, and various definitions were reviewed. Additionally, the index for pediatric CD and the index intended to evaluate small bowel lesions, such as indices related to capsule endoscopy (CE) or MRI, are described independently from the results of the research.
Flow diagram of the study selection process for CD. We performed an electronic search using PubMed. The survey period was from January 2009 to December 2017. The search string we used included “Crohn’s disease” and “clinical trial” as key words. We carefully read the studies that were extracted, and 405 studies were considered to have been evaluated by an index
Results of electronic research for CD
In terms recent (2009–2017) of CIs, the Crohn’s Disease Activity Index (CDAI) (67.4%), Harvey-Bradshaw index (Simple CDAI) (16.2%), Pediatric CDAI (11.1%), and others, were the most adopted indices. In terms of EI, the Simple Endoscopic Score for Crohn’s disease (SES-CD) (38.8%), Crohn’s Disease Endoscopic Index of Severity (CDEIS) (34.1%), and Rutgeerts Score (34.1%) were the most adopted indices. In terms of indices related to CE, the CE Crohn’s Disease Activity Index (CECDAI) (4.7%) and the Lewis Score (Capsule Endoscopy Score) (2.4%) were adopted. In terms of indices related to MRI, the Van Assche Index, Magnetic Resonance Index of Activity (MaRIA) Score, MRI Enterography Global Score (MEGS), and Magnetic Resonance Enterocolonography (MREC) Score were adopted at almost the same frequency. In terms of indices related to PROs or HR-QoL, the Inflammatory Bowel Disease Questionnaire (IBDQ) (72.5%) and Short Form-36 Health Survey Questionnaire (SF-36) (10.1%) were adopted.
In the section on CIs, CDAI and the Harvey-Bradshaw Index (Simple CDAI) are described. In the section on EIs, SES-CD, CDEIS, and Rutgeerts scores are described. Additionally, the significance of PROs or HR-QoL was evaluated. As the indices for evaluating small bowel lesions, the CECDAI and Lewis scores were described as the indices related to CE. MaRIA and MREC were described as the indices related to MRI.
Clinical Index for CD
Crohn’s Disease Activity Index (Supplementary Table 12)
This index was developed by the National Cooperative Crohn’s Disease Study (NCCDS) to evaluate the efficacy of placebo-controlled prednisone, sulfasalazine, and azathioprine on CD patients [62]. In 1976, eight variable items currently being applied then were selected from a study by multiple regression analysis [63]. In 1979, a re-evaluation of CDAI was performed [64]. Furthermore, a validation study of the selected eight variables was performed on cases attended by the NCCDS and TAS Study, a trial of sulfasalazine as adjunctive therapy for CD patients [65]. As a result, the similarity was shown in two multiple regression analyses, and it was clarified that the selected eight variable items were suitable for the evaluation of CD. For these reasons, the validity of the CDAI was rigorously verified and is currently positioned as the gold standard in CD evaluation, demonstrating its extremely high acceptance rate in clinical trials.
Problems included in CDAI and alternative indices
CDAI is a useful index, but it still has a number of problems [5, 61, 66,67,68]. (1) There are evaluation items that may vary from subject to subject, such as general condition and abdominal pain, which may affect reliability and validity. (2) The index cannot be calculated in the case of ileostomy or colostomy. (3) Retrospective evaluation may not accurately evaluate CD symptoms. (4) The standard body weight required by the evaluation was not clearly defined. (5) To unify the weighting of the scores among subjects, it is necessary to fully explain how to describe the scores; for example, the general condition is defined as good before the onset. (6) The severity of fistulas and stenosis cannot be accurately assessed by the index. (7) Finally, correlations between endoscopic activity and biomarkers are poor. Therefore, the following several indices are attracting attention as alternative indices of CDAI [67, 68].
Harvey-Bradshaw Index (Supplementary Table 13)
CDAI requires seven days to evaluate and is complicated because it consists of many items, including clinical tests. Harvey et al. developed this index to solve the complexity of CDAI [69]. The Harvey–Bradshaw Index (also known as Simple CDAI) consists of five evaluation items; three subjective clinical symptoms on the previous day, and two physical examination findings at the time of evaluation. Although it is easier to calculate than CDAI, it shows a high correlation with CDAI, and its acceptance rate in recent clinical trials is high. However, its problem is that its resulting score greatly depends on the number of liquid stools passed per day, so a CD patient with an increased number of liquid stools with intestinal resection or irritable bowel syndrome is likely to show a higher score that is disproportionate to the actual disease activity [70].
Evaluation significance of patient-reported outcomes or health-related quality of life
PROs are attracting attention as alternatives to CDAI [67, 68]. PROs provide clues as to what the patient feels as an important problem without the burden of undergoing examinations, leading to improvement in the HR-QoL of the patient [71, 72]. Recently, along with CR, achievement of living remission, such as normalized HR-QoL or absence of disability, has come to be regarded as a therapeutic target, which various PROs have presented [70]. Table 6 summarizes 69 reports in which the index related to PROs or HR-QoL was used in a recent clinical trial of CD. Among them, the IBDQ [73] was most often adopted, which was followed by the SF-36 [74]. In cases wherein disease activity of CD is low, PROs may lead to be high if psychological disorder or irritable bowel syndrome coexist with CD. Therefore, it should be noted that there are some cases in which the score of the index related to PROs or HR-QoL does not necessarily reflect disease activity [17].
Endoscopic Index for CD
Crohn’s Disease Endoscopic Index of Severity (Supplementary Table 14)
Mary et al. developed this index to standardize the endoscopic severity assessment of CD [75]. Based on endoscopic data, “global evaluation of lesion severity (GELS)” was set as dependent variable, and “average surface involved by the disease”, “average surface involved by ulcerations only”, “present, non-ulcerated, and ulcerated stenosis”, and “ number of segments exhibiting the lesion divided by the number of explored segments” were set as independent variables. Multiple regression analysis then determined six coefficients, including all six variables. The correlation between CDEIS and GELS was evaluated twice, and both showed a high correlation. Additionally, there was a high correlation observed between CDEIS and GELS for changes in patients who underwent endoscopy twice (first time: clinically active period; second time: 3–5 weeks after taking oral prednisone). Thus, CDEIS is a well-validated EI.
Simple Endoscopic Score for Crohn’s Disease (Supplementary Table 15)
CDEIS is an EI whose validity has been verified, but its calculation remains to be complicated and not necessarily easy to apply in daily clinical practice or clinical trials. Therefore, Daperno et al. developed SES-CD to create an EI that is easy to calculate and also highly valid [76]. The evaluation items of the CDEIS were referred to, and “size of ulcers”, “ulcerated surface”, “affected surface”, and “presence of narrowing” were selected as variables in SES-CD. Although it is a simpler index than is CDEIS, it is still not easy to use in daily clinical practice in terms of scoring complexity. Additionally, there are some reports pointing out difference between SES-CD and clinical disease activity [77,78,79].
Rutgeerts Score (Supplementary Table 16)
Rutgeert et al. developed and applied this index to confirm prognostic relapse factors in CD patients who underwent ileocecal resection [80]. The subjects were patients with CD who underwent ileocecal resection or right hemicolectomy and were evaluated up to 30 cm from the anastomotic site with endoscopy. The risk of re-surgery was evaluated by performing univariate analysis and regression analysis with the stepwise method for various patient backgrounds and endoscopic findings. The postoperative endoscopic findings and preoperative disease activity were shown to have a strong relationship statistically. Based on this result, they concluded that the endoscopic activity of the anastomotic site at one year after surgery most reflects the prognosis, and classified the endoscopic severity of the anastomotic site from i0 to i4.
Index for anal lesions on CD
Although CDAI is a frequently used index, it cannot reflect symptoms caused by anal lesions, which have a great effect on HR-QoL in perforative CD [63, 64]. Therefore, an evaluation using an independent index for anal lesions of CD is desirable. Considering these, this section describes perianal CDAI.
Perianal Crohn’s Disease Activity Index (Supplementary Table 17)
Irvine et al. developed this index to independently and objectively evaluate anal lesions on CD [81]. The evaluation items consisted of five items, and in addition to the clinical evaluation items, HR-QoL was evaluated by the patient regarding pain and restriction of sexual activity. Losco et al. reported that the cutoff value of 4 maximizes the sensitivity and specificity of PDAI (sensitivity: 87%, specificity: 81%) [82].
Index for evaluating small intestinal lesion of CD
Small intestinal lesions are frequently observed in patients with CD. However, it is not easy to evaluate small intestinal lesions, mainly for anatomical reasons. However, balloon-assisted enteroscopy (BAE) and capsule endoscopy (CE) have recently emerged as new endoscopic modalities and are used to evaluate the small intestinal mucosal severity of CD. Additionally, as shown in Table 5, recent clinical trials have also applied indices related to MRI, which can evaluate the transmural severity of CD. Each modality has its own advantages and disadvantages, and it is not always possible to evaluate the entire small intestine with a single modality. Available modalities are therefore recommended to be used complementarily when necessary. This section describes indices related to CE (Lewis Score, CECDAI) and indices related to MRI (MaRIA Score, MREC Score).
Lewis Score (Supplementary Table 18)
Gralnek et al. developed this index to score inflammatory changes seen in the small intestine based on CE findings [83]. The three findings of villous edema, ulcer, and stenosis, which showed a high concordance rate among observers, were defined as the evaluation items for inflammatory changes. The small intestine transit time of the CE is divided into three equal parts (1st–3rd tertile). Based on this division, the Lewis Score is the sum of the highest score, which is the sum of the villus appearance score and the ulcer score for each tertile, and the stenosis score for the entire small intestine. In the original article, < 135 was defined as the threshold to consider remission. However, it should be noted that this index is not specialized for CD, but for non-specific inflammatory findings found in other diseases.
Capsule Endoscopy Crohn’s Disease Activity Index (Supplementary Table 19)
Gal et al. developed this index to evaluate the activity of CDs using CE [84]. This index consists of three items: inflammation score, extent of disease score, and narrowing (stricture). Small bowel transit time (SBTT) was divided into two equal parts. CECDAI is a quantitative score that evaluates these three items and summarizes the scores. In the original article, the total scores of four physicians for the same patient showed similarity, and the correlation coefficient of Spearman was also high (0.8–0.93, p < 0.001). However, although CE itself is limited, capsule retention and incomplete observation of the entire small intestine (approximately 20%) have been identified. The definition of MH or endoscopic severity was not presented in the original article.
CECDAIic Score (Supplementary Table 20)
Compared to ileocolonoscopy, although a poorer completion rate due to insufficient bowel preparation and the risk of retention due to stricture were mentioned, colon CE is an expected alternative examination for ileocolonoscopy in the evaluation of the colonic mucosa [85]. In 2018, Niv et al. developed CECDAIic, in which the evaluation target site of CECDAI was “extended” to the colon [86].
Magnetic Resonance Index of Activity Score (Supplementary Table 21)
Rimola et al. developed this index to evaluate CD activity using MRI [87]. It was confirmed that the score showed a good correlation with HBI and CDEIS. Although the cutoff value and definition have not been clearly defined, if MH is defined as MRA score < 7, sensitivity and specificity are 85% and 78%, respectively [88]. Additionally, although the original study examined the large intestine and the terminal ileum only, sensitivity and specificity were observed at 87% and 86%, respectively, for the diagnostic ability of MH upon the application of the index [89]. In the original article, the definition was not clearly determined, but “MaRIA Score ≤ 7” was used as the definition of inactive CD [5].
Magnetic Resonance Enterocolonography Score (Supplementary Table 22)
Takenaka et al. developed this index to compare MRI and endoscopic findings [90]. Unlike the MaRIA score, the MREC score rates each finding and eliminates complicated calculations. The MREC Score showed a good correlation with SES-CD and modified Rutgeerts score, slightly weak correlation with CDAI, and no statistically significant correlation with CRP. Thus, this index is considered a better proxy of the endoscopic findings than is the disease activity. However, it has been pointed out that its ability to identify stenosis is inferior to that of BAE. The definition of transmural remission or activity of CD is not presented in the original article.
Index for pediatric CD
Pediatric CD cases, compared to adult CD cases, have more extensive and severe lesions at diagnosis, show a higher frequency of progressive advances, and show higher proportions of small intestinal lesions, and growth disorder is observed in 10–40% of patients with pediatric CD at diagnosis [45, 46, 91]. Therefore, in the clinical practice for managing pediatric CD, it is necessary to consider growth disorders and the risk of aggravation in a short period of time in children compared to adults [91]. This section describes pediatric CDAI, which was developed to address these problems.
Pediatric Crohn’s Disease Activity Index (Supplementary Table 23)
Hyams et al. developed this index to match the characteristics of pediatric CD [92]. The concordance rate between observers was evaluated, and a good concordance rate (r = 0.81) was observed. It also showed a good correlation with PGA (r = 0.77) and the modified Harvey-Bradshaw Score (r = 0.86). The characteristic of this index is that it reflects growth factors, such as changes in height and weight, in addition to symptoms and laboratory findings. This index should be applied at the time of CD diagnosis, and recovery of growth disorders after diagnosis should be evaluated using a growth rate curve. Moreover, its correlation with SES-CD is poor to fair [5].
Definitions of each index
This section defines the indices described in this review. Additionally, the definitions recommended in STRIDE-II are underlined.
Clinical Index for UC
With the advent of various therapeutic agents, treatment of UC is advancing day by day; both treatment options and treatment diversity are increasing. In terms of determining therapeutic effectiveness for severe cases, indices that can reflect short-term response sharply tend to be applied more frequently. In contrast, evaluation of short-term response is not important for drugs, such as 5-ASA or immuno-modulators, and short-term fluctuations are not usually required for the index. In other words, the choice of CI and whether CR or clinical response is defined depends on the type of therapeutic agent being used as well as the characteristics of the clinical trial. Therefore, to utilize CI, its characteristics and precisely how to use it must be understood. Therefore, this section summarizes the definitions frequently used in clinical trials of each of the CIs outlined. The results are presented in Table 7.
Endoscopic Index for UC
In many clinical trials of UC, in particular, new drugs, ER or MH are listed as primary or secondary evaluation endpoints. However, its definition currently differs per clinical trial. For example, MH is defined as 0 or 1 in MES in many papers, but recently, it has been required to aim for MES 0 to improve the long-term clinical course [5, 13,14,15]. Additionally, the definition of MH by UCEIS, whose validity has been verified and proposed, is increasingly being adopted. Therefore, this section summarizes the definitions frequently used in clinical trials for each of the outlined EIs. The results are presented in Table 8.
Clinical Index for CD
CDAI can be said to be the champion index of CI for CD, and has it been adopted in most clinical trials. The definition of CR was defined as CDAI < 150, which now appears to be the gold standard. However, definitions of response, relapse, and active are defined but used in various ways in different trial. Therefore, especially for CDAI, the results for these definitions are described in detail. Additionally, the disease characteristics of CD include many cases of onset at a young age with a high rate of anal lesions. Therefore, pediatric CDAI and perianal CDAI are highly needed as separate evaluation indices independent of CDAI. Therefore, this section summarizes the definitions frequently used in clinical trials of each of the CIs outlined. The results are presented in Table 9.
Endoscopic Index for CD
In CD, an evaluation method for colorectal lesions has been established, but there remains to be no consensus on the evaluation of small intestinal lesions. Definitions of MH and transluminal healing using CT and MRI have also been proposed [6]. Considering these, this section summarizes the definition of ER or MH used in clinical trials to better understand the above trends and issues. The results are presented in Table 10.
Summary and conclusion
This survey clarified the use of CIs and EIs in recent clinical trials for IBD. The index related to HR-QoL or PROs and the index using new modalities, such as CE and MRI, were used in certain frequencies depending on the application. Based on the survey results, we outlined the main indices deemed to be most useful in clinical practice and clinical trial planning. Additionally, the definition of CR and MH was explained as much as possible. Recently, the treatment of IBD has progressed, and along with this, methods of evaluating its activity based on treatment targets and long-term perspectives have also changed. At present, MH remains to be a valid therapeutic target, and endoscopic evaluation also remains to be emphasized in managing IBD. However, frequent monitoring using invasive endoscopy is not feasible. Therefore, indices and biomarkers that correlate with endoscopic severity were investigated. Alternatively, as suggested by STRIDE-II, the evaluation method based on the concept of T2T, which achieves the treatment target according to the treatment timing, is being questioned. In other words, the targets are improvement of symptoms and PRO in the short term, normalization of biomarkers in the intermediate term, and ER and HR in the long term. Corresponding indices and biomarkers were selected for each target. It is important for physicians engaged in managing IBD to know the characteristics of each index and biomarker, as well as to select and utilize the appropriate index and biomarker that match their treatment targets in clinical settings or clinical trials.
Abbreviations
- IBD:
-
Inflammatory bowel disease
- CI:
-
Clinical index
- EI:
-
Endoscopic index
- MH:
-
Mucosal healing
- PH:
-
Pathological healing
- T2T:
-
Treat to target
- CRP:
-
C-reactive protein
- FC:
-
Fecal calprotectin
- UC:
-
Ulcerative colitis
- CD:
-
Crohn’s disease
- DAI:
-
Disease activity index
- CAI:
-
Clinical activity index
- SCCAI:
-
Simple Clinical Colitis Activity Index
- MES:
-
Mayo Endoscopic Sub-score
- UCEIS:
-
Ulcerative Colitis Endoscopic Index of Severity
- CR:
-
Complete response
- PR:
-
Partial response
- UCCS:
-
Ulcerative Colitis Clinical Score
- PGA:
-
Physician’s Global Assessment
- p-MS:
-
Partial Mayo Score
- ER:
-
Endoscopic remission
- PUCAI:
-
Pediatric UC Activity Index
- PDAI:
-
Pouchitis Disease Activity Index
- MRI:
-
Magnetic resonance imaging
- PROs:
-
Patient-reported outcomes
- HR-QoL:
-
Health-related quality of life
- CE:
-
Capsule endoscopy
- CDAI:
-
Crohn’s Disease Activity Index
- SES-CD:
-
Simple Endoscopic Score for CD
- CDEIS:
-
Crohn’s Disease Endoscopic Index of Severity
- CECDAI:
-
CE Crohn’s Disease Activity Index
- MaRIA:
-
Magnetic Resonance Index of Activity
- MEGS:
-
MRI Enterography Global Score
- MREC:
-
Magnetic resonance enterocolonography
- IBDQ:
-
Inflammatory Bowel Disease Questionnaire
- SF-36:
-
Short Form-36 Health Survey Questionnaire
- NCCDS:
-
National Cooperative Crohn’s Disease Study
- GELS:
-
Global evaluation of lesion severity
- BAE:
-
Balloon-assisted enteroscopy
- CE:
-
Capsule endoscopy
References
Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology. 2015;148:37–51.
Walsh AJ, Bryant RV, Travis SPL. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol. 2016;13:567–79.
Bressler B, Marshall JK, Bernstein CN, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148:1035–58.
Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–38.
Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2020. https://doi.org/10.1053/j.gastro.2020.12.031.
Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:978–85.
Jairath V, Zou GY, Parker CE, et al. Placebo response and remission rates in randomised trials of induction and maintenance therapy for ulcerative colitis. Cochrane Datab Syst Rev. 2017. https://doi.org/10.1002/14651858.CD011572.pub2.
Mohammed Vashist N, Samaan M, Mosli MH, et al. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Datab Syst Rev. 2018. https://doi.org/10.1002/14651858.CD011450.pub2.
D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–86.
Su CY, Lewis JD, Goldberg B, et al. A meta-analysis of the placebo rates of remission and response in clinical trials of active ulcerative colitis. Gastroenterology. 2007;132:516–26.
Ilnyckyj A, Shanahan F, Anton PA, et al. Quantification of the placebo response in ulcerative colitis (comment). Gastroenterology. 1997;112:1854–8.
Hirai F, Matsui T, Aoyagi K, et al. Validity of activity indices in ulcerative colitis: comparison of clinical and endoscopic indices. Dig Endosc. 2010;22:39–44.
Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–9.
Sutherland LR, Martin F, Greer S, et al. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894–8.
Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298(6666):82–6.
Walmsley RS, Ayres RCS, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43:29–32.
Jowett SL, Seal CJ, Phillips E, et al. Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol. 2003;38:164–71.
Lichtiger S, Present DH. Preliminary report: cyclosporin in treatment of severe active colitis. Lancet. 1990;336:16–9.
Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–5.
Barreiro-de Acosta M, Vallejo N, de la Iglesia D, et al. Evaluation of the risk of relapse in ulcerative colitis according to the degree of mucosal healing (Mayo 0 vs 1): A Longitudinal Cohort Study. J Crohns Colitis. 2019;10:13–9.
Vuitton L, Peyrin-Biroulet L, Colombel JF, et al. Defining endoscopic response and remission in ulcerative colitis clinical trials: an international consensus. Aliment Pharmacol Ther. 2017;45:801–13.
Yokoyama K, Kobayashi K, Mukae M, et al. Clinical study of the relation between mucosal healing and long-term outcomes in ulcerative colitis. Gastroenterol Res Pract. 2013. https://doi.org/10.1155/2013/192794.
Mazzuoli S, Guglielmi FW, Antonelli E, et al. Definition and evaluation of mucosal healing in clinical practice. Dig Liver Dis. 2013;45:969–77.
Carvalho PB, de Castro DF, Rosa B, et al. Mucosal healing in ulcerative colitis: when zero is better. J Crohns Colitis. 2016;10:20–5.
Nakarai A, Kato J, Hiraoka S, et al. Prognosis of ulcerative colitis differs between patients with complete and partial mucosal healing, which can be predicted from the platelet count. World J Gastroenterol. 2014;20:18367–74.
Travis SP, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the ulcerative colitis endoscopic index of severity (UCEIS). Gut. 2012;61:535–42.
Travis SP, Schnell D, Krzeski P, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013;145:987–95.
Ikeya K, Hanai H, Sugimoto K, et al. The ulcerative colitis endoscopic index of severity more accurately reflects clinical outcomes and long-term prognosis than the Mayo endoscopic score. J Crohns Colitis. 2016;10:286–95.
Arai M, Naganuma M, Sugimoto S, et al. The Ulcerative Colitis Endoscopic Index of Severity is useful to predict medium- to long-term prognosis in ulcerative colitis patients with clinical remission. J Crohns Colitis. 2016;10:1303–9.
Xie T, Zhang T, Ding C, et al. Ulcerative colitis endoscopic index of severity (UCEIS) versus Mayo endoscopic score (MES) in guiding the need for colectomy in patients with acute severe colitis. Gastroenterol Rep (Oxf). 2018;6:38–44.
Mosli MH, Parker CE, Nelson SA, et al. Histologic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Datab Syst Rev. 2017. https://doi.org/10.1002/14651858.CD011256.pub2.
Jong DC, Lowënberg M, Koumoutsos I, et al. Validation and investigation of the operating characteristics of the ulcerative colitis endoscopic index of severity. Inflamm Bowel Dis. 2019;25:937–44.
Jiménez MB, Hergueta-Delgado P, Rodríguez BG, et al. Comparison of the mayo endoscopy score and the ulcerative colitis endoscopy index of severity and the ulcerative colitis colonoscopy index of severity. Endosc Int Open. 2021;9:E130–6.
Römkens TEH, Kranenburg P, Tilburg AV, et al. Assessment of histological remission in ulcerative colitis: discrepancies between daily practice and expert opinion. J Crohn’s Colitis. 2018;12:425–31.
Geboes K, Rutgeerts P, Olson A, et al. Infliximab results in reduction of inflammation and inflammatory markers in the mucosa of ulcerative colitis patients: the ACT 1 trial (abstr). Am J Gastroenterol. 2005;100:S287.
Lemmens B, Arijs I, Van Assche G, et al. Correlation between the endoscopic and histologic score in assessing the activity of ulcerative colitis. Inflamm Bowel Dis. 2013;19:1194–201.
Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med. 1961;30:393–407.
Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174–8.
Riley SA, Mani V, Goodman MJ, et al. Comparison of delayed-release 5-aminosalicylic acid (mesalazine) and sulfasalazine as maintenance treatment for patients with ulcerative colitis. Gastroenterology. 1988;94:1383–9.
Ardizzone S, Maconi G, Russo A, et al. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55:47–53.
Paoluzi OA, Pica R, Marcheggiano A, et al. Azathioprine or methotrexate in the treatment of patients with steroid-dependent or steroid-resistant ulcerative colitis: results of an open-label study on efficacy and tolerability in inducing and maintaining remission. Aliment Pharmacol Ther. 2002;16:1751–9.
Geboes K, Riddle R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–9.
Tursi A, Elisei W, Picchio M, et al. Effectiveness of adalimumab for ambulatory ulcerative colitis patients after failure of infliximab treatment: a first “real-life” experience in primary gastroenterology centers in Italy. Ann Gastroenterol. 2014;27:369–73.
Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95.
Bequet E, Sarter H, Fumery M, et al. Incidence and phenotype at diagnosis of very-early-onset compared with later-onset paediatric inflammatory bowel disease: a population-based study [1988-2011]. J Crohns Colitis. 2017;11:519–26.
Birimberg-Schwartz L, Zucker DM, Akriv A, et al. Development and validation of diagnostic criteria for IBD subtypes including IBD-unclassified in children: a multicenter study from the pediatric IBD porto group of ESPGHAN. J Crohns Colitis. 2017;11:1078–84.
Ruemmele FM, Turner D. Differences in the management of pediatric and adult onset ulcerative colitis-lessons from the joint ECCO and ESPGHAN consensus guidelines for the management of pediatric ulcerative colitis. J Crohns Colitis. 2014;8:1–4.
Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatrtic ulcerativecolitis activity index. Gastroenterology. 2007;133:423–32.
Turner D, Walsh CM, Benchimol EI, et al. Severe paediatric ulcerative colitis: incidence, outcomes and optimal timing for second-line therapy. Gut. 2008;57:331–8.
Turner D, Mack D, Leleiko N, et al. Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010;138:2282–91.
Abou KM, Boutros M, Nedjar H, et al. Incidence rates and predictors of colectomy for ulcerative colitis in the era of biologics: results from a provincial database. J Gastrointest Surg. 2018;22:124–32.
Kuehn F, Hodin RA. Impact of modern drug therapy on surgery: ulcerative colitis. Visc Med. 2018;34:426–31.
Olén O, Askling J, Sachs MC, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964–2014. Gut. 2020;69:453–61.
Ahmed A, Laverty AA, Alexakis C, et al. Changing nationwide trends in endoscopic, medical and surgical administrations for inflammatory bowel disease: 2003–2013. BMJ Open Gastroenterol. 2018. https://doi.org/10.1136/bmjgast-2017-000191.
Rutter MD, Saunders BP, Wilkinson KH, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–8.
Ikeuchi H, Uchino M, Matsuoka H, et al. Surgery for ulcerative colitis in 1,000 patients. Int J Colorectal Dis. 2010;25:959–65.
Sandborn WJ, Tremaine WJ, Batts KP, et al. Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc. 1994;69:409–15.
Yuho S, Matsui T, Yano Y, et al. Long-term course of Crohn’s disease in Japan: Incidence of complications, cumulative rate of initial surgery, and risk factors at diagnosis for initial surgery. J Gastroenterol Hepatol. 2015;30:1713–9.
Higashi D, Katsuno H, Kimura H, et al. Current state of and problems related to cancer of the intestinal tract associated with Crohn’s disease in Japan. Anticancer Res. 2016;36:3761–6.
Khanna R, Nelson SA, Feagan BG, et al. Endoscopic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst Rev. 2016. https://doi.org/10.1002/14651858.CD010642.pub2.
Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122:512–30.
Summers RW, Switz DM, Sessions JT Jr, et al. National cooperative Crohn’s disease study: results of drug treatment. Gastroenterology. 1979;77:847–69.
Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. Natl Cooper Crohn’s Dis Study Gastroenterol. 1976;70:439–44.
Best WR, Becktel JM, Singleton JW, et al. Rederived values of the eight coefficients of the Crohn’s disease activity index (CDAI). Gastroenterology. 1979;77:843–6.
Singleton JW, Summers RW, Kern F Jr, et al. A trial of sulfasalazine as adjunctive therapy in Crohn’s disease. Gastroenterology. 1979;77:887–97.
Sostegni R, Daperno M, Scaglione N, et al. Review article; Crohn’s disease: monitoring disease activity. Aliment Phamacol Ther. 2003;17:11–7.
Lewis JD, Rutgeerts PR, Feagan BG, et al. Correlation of stool frequency and abdominal pain measures with simple endoscopic score for Crohn’s disease. Inflamm Bowel Dis. 2020;26:304–31.
Jong MJ, Huibregtse R, Masclee AM, et al. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: A systematic review. Clin Gastroenterol Hepatol. 2018;16:648–63.
Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514.
Sturm A, Maaser C, Calabrese E, et al. ECCO-ESGAR Guideline for diagnostic assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;26:273–84.
Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s relapse prevention trial study group. Gastroenterology. 1994;106:287–96.
Turner D, Griffiths AM, Mack D, et al. Assessing disease activity in ulcerative colitis: patients or their physicians? Inflamm Bowel Dis. 2010;16:651–6.
Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804–10.
Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306:1437–40.
Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digesti (f GETAID). Gut. 1989;30:983–9.
Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–12.
Jones J, Loftus EV Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;11:1218–24.
Falvey JD, Hoskin T, Meijer B, et al. Disease activity assessment in IBD. Inflamm Bowel Dis. 2015;21:824–31.
Khanna R, Bouguen G, Feagan BG, et al. A systematic review of measurement of endoscopic disease activity and mucosal healing in Crohn’s disease: recommendations for clinical trial design. Inflamm Bowel Dis. 2014;20:1850–61.
Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–63.
Irvine EJ. Usual therapy improves perianal Crohn’s disease as measured by a new disease activity index. McMaster IBD Study Group. J Clin Gastroenterol. 1995;20:27–32.
Losco A, Viganò C, Conte D, et al. Assessing the activity of perianal Crohn’s disease: comparison of clinical indices and computer-assisted anal ultrasound. Inflamm Bowel Dis. 2009;15:742–9.
Gralnek IM, Defranchis R, Seidman E, et al. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146–54.
Gal E, Geller A, Fraser G, et al. Assessment and validation of the new capsule endoscopy Crohn’s disease activity index (CECDAI). Dig Dis Sci. 2008;53:1933–7.
Papalia I, Tjandra D, Quah S, et al. Colon cacpsule endoscopy in the assessment of mucosal healing in Crohn’s disease. Inflamm Bowel Dis. 2021;15:S25–32.
Niv Y, Gal E, Gabovitz V, et al. Capsule endoscopy Crohn’s disease activity index (CECDAIic or Niv Score) for the small bowel and colon. J Clin Gastroenterol. 2018;52:45–9.
Rimola J, Rodriguez S, García-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113–20.
Ordàs I, Rimola J, Rodrìguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology. 2014;146:374–82.
Takenaka K, Ohtsuka K, Kirazume Y, et al. Correlation of the endoscopic and magnetic resonance scoring system in the deep small intestine in Crohn’s disease. Inflamm Bowel Dis. 2015;21:1832–8.
Takenaka K, Ohtsuka K, Kitazume Y, et al. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn’s disease. Gastroenterology. 2014;147:334–42.
Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8:1179–207.
Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–47.
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76.
Gustavsson A, Jarnerot G, Hertervig E, et al. Clinical trial: colectomy after rescue therapy in ulcerative colitis—3-year follow-up of the Swedish-Danish controlled infliximab study. Aliment Pharmacol Ther. 2010;32:984–9.
Cabriada JL, Ibargoyen N, Hernandez A, et al. Sustained remission after steroids and leukocytapheresis induced response in steroid-dependent ulcerative colitis. Dig Liver Dis. 2010;42:432–5.
Suzuki T, Mizoshita T, Tanida S, et al. The efficacy of maintenance therapy after remission induction with tacrolimus in ulcerative colitis with and without previous tumor necrosis factor-α inhibitor. JGH Open. 2019;3:217–23.
Sandborn WJ, Assche GV, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–65.
Colombel JF, Sandborn WJ, Ghosh S, et al. Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: Data from ULTRA 1, 2, and 3. Am J Gastroenterol. 2014;109:1771–80.
Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.
Suzuki Y, Motoya S, Hanai H, et al. Four-year maintenance treatment with adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2017;52:1031–40.
Sands BE, Biroulet LP, Loftus EV, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381:1215–26.
Hibi T, Imai Y, Senoo A, et al. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: a phase 3, double-blind, randomized, placebo-controlled study-(PURSUIT-J study). J Gastroenterol. 2017;52:1101–11.
Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–24.
Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–36.
Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J MED. 2013;369:699–710.
Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780–7.
Rispo A, Testa A, De Palma GD, et al. Different profile of efficacy of thiopurines in ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2015;21:2570–5.
Dai C, Liu WX, Jiang M, et al. Mucosal healing did not predict sustained clinical remission in patients with IBD after discontinuation of one-year infliximab therapy. PLoS ONE. 2014;9:e110797.
Ogata H, Matsui T, Nakamura M, et al. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut. 2006;55:1255–62.
Yamamoto T, Shimoyama T, Umegae S, et al. Tacrolimus vs. anti-tumour necrosis factor agents for moderately to severely active ulcerative colitis: a retrospective observational study. Aliment Pharmacol Ther. 2016;43:705–16.
Feagan BG, Sandborn WJ, Dhaens G, et al. The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology. 2013;145:149–57.
Kamm MA, Sandborn WJ, Gassull M, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterol. 2007;132:66–75.
Ogata H, Ohori A, Nishino H, et al. Comparison of efficacies of once-daily dose multimatrix mesalazine and multiple-dose mesalazine for the maintenance of remission in ulcerative colitis: a randomized, double-blind study. Intest Res. 2017;15:358–67.
Prantera C, Kohn A, Campieri M, et al. Clinical trial: ulcerative colitis maintenance treatment with 5-ASA: a 1-year, randomized multicentre study comparing MMX® with Asacol®. Aliment Pharmacol Ther. 2009;30:908–18.
Ogata H, Kato J, Hirai F, et al. Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2012;18:803–8.
Tursi A, Elisei W, Picchio M, et al. Managing ambulatory ulcerative colitis patients with infliximab: a long-term follow-up study in primary gastroenterology centers. Eur J Intern Med. 2014;25:757–61.
Gibson PR, Fixa B, Pekárková B, et al. Comparison of the efficacy and safety of Eudragit-L-coated mesalazine tablets with ethylcellulose-coated mesalazine tablets in patients with mild to moderately active ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1017–26.
Kruis W, Bar-Meir S, Feher J, et al. The optimal dose of 5-aminosalicylic acid in active ulcerative colitis: a dose-finding study with newly developed mesalamine. Clin Gastroenterol Hepatol. 2003;1:36–43.
Endo K, Onodera M, Shiga H, et al. A comparison of short- and long-term therapeutic outcomes of infliximab –versus tacrolimus-based strategies for steroid-refractory ulcerative colitis. Gastroenterol Res and Pract. 2016. https://doi.org/10.1155/2016/3162595.
Miyoshi J, Matsuoka K, Inoue N, et al. Mucosal healing with oral tacrolimus is associated with favorable medium- and long-term prognosis in steroidrefractory/dependent ulcerative colitis patients. J Crohns Colitis. 2013;7:e609–14.
Kato K, Ohkusa T, Terao S, et al. Adjunct antibiotic combination therapy for steroid-refractory or -dependent ulcerative colitis: an open-label multicentre study. Aliment Pharmacol Ther. 2014;39:949–56.
Turner D, Hyams J, Markowitz J, et al. Appraisal of the pediatric ulcerative colitis activity index (PUCAI). Inflamm Bowel Dis. 2009;15:1218–23.
Christensen B, Gibson PR, Micic D, et al. Safety and efficacy of combination treatment with calcineurin inhibitors and vedolizumab in patients with refractory inflammatory bowel disease. Clin Gastroenterol Hepatol. 2019;17:486–93.
Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–201.
Yamamoto T, Umegae S, Matsumoto K. Mucosal healing in patients with ulcerative colitis during a course of selective leukocytapheresis therapy: a prospective cohort study. Inflamm Bowel Dis. 2010;16:1905–11.
Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;141:1102–11.
Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebocontrolled phase 2 study. Lancet. 2017;389:1699–709.
Feagan BG, Panés J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol. 2018;3:671–80.
Candy S, Wright J, Gerber M, et al. A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut. 1995;37:674–8.
Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory crohn’s disease. N Engl J Med. 2012;367:1519–28.
Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for crohn’s disease. N Engl J Med. 2016;375:1946–60.
Gross V, Andus T, Ecker KW, et al. Low dose oral pH modified release budesonide for maintenance of steroid induced remission in Crohn’s disease. The Budesonide Study Group Gut. 1998;42:493–6.
Sutherland LR, Martin F, Bailey RJ, et al. A randomized, placebo-controlled, double-blind trial of mesalamine in the maintenance of remission of Crohn’s disease. The Canadian Mesalamine for Remission of Crohn’s Disease Study Group. Gastroenterology. 1997;112:1069–77.
Gendre JP, Mary JY, Florent C, et al. Oral mesalamine (Pentasa) as maintenance treatment in Crohn’s disease: a multicenter placebo-controlled study. The Groupe d’Etudes Therapeutiques des Affections Inflammatoires Digestives (GETAID). Gastroenterology. 1993;104:435–9.
Stange EF, Modigliani R, Pena AS, et al. European trial of cyclosporine in chronic active Crohn’s disease: a 12-month study. Eur Study Group Gastroenterol. 1995;109:774–82.
Feagan BG, McDonald JW, Rochon J, et al. Low-dose cyclosporine for the treatment of Crohn’s disease. The Canadian Crohn’s relapse prevention trial investigators. N Engl J Med. 1994;330:1846–51.
Landi B, Anh TN, Cortot A, et al. Endoscopic monitoring of Crohn’s disease treatment: a prospective, randomized clinical trial. The groupe d’etudes therapeutiques des affections inflammatoires digestives. Gastroenterology. 1992;102:1647–53.
Modigliani R, Mary JY, Simon JF, et al. Clinical, biological, and endoscopic picture of attacks of Crohn’s disease. Evolution on prednisolone groupe d’etude therapeutique des affections inflammatoires digestives. Gastroenterology. 1990;98:811–8.
Cortot A, Colombel JF, Rutgeerts P, et al. Switch from systemic steroids to budesonide in steroid dependent patients with inactive Crohn’s disease. Gut. 2001;48:186–90.
D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–7.
Thomson AB, Wright JP, Vatn M, et al. Mesalazine (Mesasal/Claversal) 1placebo in the maintenance of remission of patients with Crohn’s disease. Aliment Pharmacol Ther. 1995;9:673–83.
Brignola C, Cottone M, Pera A, et al. Mesalamine in the prevention of endoscopic recurrence after intestinal resection for Crohn’s disease. Ital Cooper Study Group Gastroenterol. 1995;108:345–9.
Lochs H, Mayer M, Fleig WE, et al. Prophylaxis of postoperative relapse in Crohn’s disease with mesalamine: European cooperative Crohn’s disease study VI. Gastroenterology. 2000;118:264–73.
Caprilli R, Andreoli A, Capurso L, et al. Oral mesalazine (5-aminosalicylic acid; Asacol) for the prevention of post-operative recurrence of Crohn’s disease. Gruppo Italiano per lo Studio del Colon e del Retto (GISC). Aliment Pharmacol Ther. 1994;8:35–43.
Khhana R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial. Lancet. 2015;7:1825–34.
Acknowledgements
This study was funded and carried out within the framework of a study project undertaken by the Study Group on Intractable Diseases, the Health and Labour Sciences Research Grants (chief researchers: Dr Y. Suzuki and T. Hisamatsu) from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The idea for the article was guided by FH. The literature search and data analysis were performed by MK. The drafting and critically revising the work were performed by FH, TH, NT and MK. Study supervision: MK, FH, NT, YT, TB, KT, MN, KO, KW, TM, ME, KK, AS, KH, KF, YA, HT, AI, TH, HS, KA, YS, TH.
Corresponding author
Ethics declarations
Conflict of interest
Any financial relationship with enterprises, businesses or academic institutions in the subject matter or materials discussed in the manuscript are listed as follows; (1) those from which the authors, the spouse, partner or immediate relatives of authors, have received individually any income, honoraria or any other types of remuneration; Abbvie GK, EA Pahrma Co., Ltd, Janssen Pharmaceutical K.K, Mochida Pharmaceutical Co., Ltd, Tanabe Mitsubishi Pharmaceutical Co., Ltd, Takeda Pharmaceutical Co., Ltd, Kyorin Pharmaceutical Co., Ltd, Pfizer Japan Inc. and Kissei Pharmaceutical Co., Ltd, Nichi-iko Paermaceutical Co., Ltd, JIMRO Co., Ltd and (2) those from which the academic institutions of the authors received support (commercial/academic cooperation); Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Abbvie GK, EA Pahrma Co., Ltd, Eisai Co., Ltd, Otsuka Pharmaceutical Co., Ltd, Kissei Pharmaceutical Co., Ltd, Nippon Kayaku Co., Ltd, Mochida Pharmaceutical Co., Ltd, Tanabe Mitsubishi Pharmaceutical Co., Ltd, Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd, EP-CRSU Co,. Ltd, ZERIA Pharmaceutical Co., Ltd, Alfresa Pharma Co. Ltd, JIMRO Co., Ltd. Daichi-Sankyo Co., Ltd, Kyorin Pharmaceutical Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kishi, M., Hirai, F., Takatsu, N. et al. A review on the current status and definitions of activity indices in inflammatory bowel disease: how to use indices for precise evaluation. J Gastroenterol 57, 246–266 (2022). https://doi.org/10.1007/s00535-022-01862-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-022-01862-y