Abstract
Background
Although endoscopic ultrasound (EUS) is reported to be suitable for determining the layer from which subepithelial lesions (SELs) originate, it is difficult to distinguish gastrointestinal stromal tumor (GIST) from non-GIST using only EUS images. If artificial intelligence (AI) can be used for the diagnosis of SELs, it should provide several benefits, including objectivity, simplicity, and quickness. In this pilot study, we propose an AI diagnostic system for SELs and evaluate its efficacy.
Methods
Thirty sets each of EUS images with SELs ≥ 20 mm or < 20 mm were prepared for diagnosis by an EUS diagnostic system with AI (EUS-AI) and three EUS experts. The EUS-AI and EUS experts diagnosed the SELs using solely the EUS images. The concordance rates of the EUS-AI and EUS experts’ diagnoses were compared with the pathological findings of the SELs.
Results
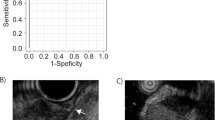
The accuracy, sensitivity, and specificity for SELs < 20 mm were 86.3, 86.3, and 62.5%, respectively for the EUS-AI, and 73.3, 68.2, and 87.5%, respectively, for the EUS experts. In contrast, accuracy, sensitivity, and specificity for SELs ≥ 20 mm were 90.0, 91.7, and 83.3%, respectively, for the EUS-AI, and 53.3, 50.0, and 83.3%, respectively, for the EUS experts. The area under the curve for the diagnostic yield of the EUS-AI for SELs ≥ 20 mm (0.965) was significantly higher than that (0.684) of the EUS experts (P = 0.007).
Conclusion
EUS-AI had a good diagnostic yield for SELs ≥ 20 mm. EUS-AI has potential as a good option for the diagnosis of SELs.



Similar content being viewed by others
References
Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20–3.
Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293–301.
Crosby JA, Catton CN, Davis A, et al. Malignant gastrointestinal stromal tumors of the small intestine: a review of 50 cases from a prospective database. Ann Surg Oncol. 2001;8:50–9.
Casali PG, Abecassis N, Aro HT, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:68–78.
Faulx AL, Kothari S, Acosta RD, et al. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. 2017;85:1117–32.
Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3–14.
Yoshinaga S, Hilmi IN, Kwek BE, et al. Current status of endoscopic ultrasound for the upper gastrointestinal tract in Asia. Dig Endosc. 2015;27(Suppl 1):2–10.
Minoda Y, Chinen T, Osoegawa T, et al. Superiority of mucosal incision-assisted biopsy over ultrasound-guided fine needle aspiration biopsy in diagnosing small gastric subepithelial lesions: a propensity score matching analysis. BMC Gastroenterol. 2020;20:19.
Fernandez-Esparrach G, Sendino O, Sole M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: a randomized crossover study. Endoscopy. 2010;42:292–9.
Fujioka T, Kubota K, Mori M, et al. Distinction between benign and malignant breast masses at breast ultrasound using deep learning method with convolutional neural network. Jpn J Radiol. 2019;37:466–72.
Han S, Kang HK, Jeong JY, et al. A deep learning framework for supporting the classification of breast lesions in ultrasound images. Phys Med Biol. 2017;62:7714–28.
Mori Y, Kudo SE, East JE, et al. Cost savings in colonoscopy with artificial intelligence-aided polyp diagnosis: an add-on analysis of a clinical trial (with video). Gastrointest Endosc. 2020. https://doi.org/10.1016/j.gie.2020.03.3759(In press).
Kudo SE, Misawa M, Mori Y, et al. Artificial intelligence-assisted system improves endoscopic identification of colorectal neoplasms. Clin Gastroenterol Hepatol. 2019. https://doi.org/10.1016/j.cgh.2019.09.009(in press).
Mori Y, Kudo SE, Misawa M, et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: a prospective study. Ann Intern Med. 2018;169:357–66.
Seo SW, Hong SJ, Han JP, et al. Accuracy of a scoring system for the differential diagnosis of common gastric subepithelial tumors based on endoscopic ultrasonography. J Dig Dis. 2013;14:647–53.
Yegin EG, Duman DG. Small EUS-suspected gastrointestinal stromal tumors of the stomach: an overview for the current state of management. Endosc Ultrasound. 2016;5:69–77.
Tateishi U, Hasegawa T, Satake M, et al. Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr. 2003;27:792–8.
de Moura DTH, McCarty TR, Jirapinyo P, et al. EUS-guided fine-needle biopsy versus fine-needle aspiration in the diagnosis of subepithelial lesions: a large multicenter study. Gastrointest Endosc. 2020;92:108–19.
Akahoshi K, Oya M, Koga T, et al. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806–17.
Inoue T, Okumura F, Sano H, et al. Impact of endoscopic ultrasound-guided fine-needle biopsy on the diagnosis of subepithelial tumors: a propensity score-matching analysis. Dig Endosc. 2019;31:156–63.
Osoegawa T, Minoda Y, Ihara E, et al. Mucosal incision-assisted biopsy versus endoscopic ultrasound-guided fine-needle aspiration with a rapid on-site evaluation for gastric subepithelial lesions: a randomized cross-over study. Dig Endosc. 2019;31:413–21.
Akahoshi K, Oya M, Koga T, et al. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J Gastrointest Liver Dis. 2014;23:405–12.
Larghi A, Fuccio L, Chiarello G, et al. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy. 2014;46:39–45.
Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–82.
Acknowledgements
We thank Dr. Junji Kishimoto in the Kyushu University Center for Clinical and Translational Research for assisting with the statistical analysis.
Author information
Authors and Affiliations
Contributions
YM designed the study, analyzed the data, and wrote the manuscript. HO edited the manuscript. EI collected the data and critically reviewed the manuscript. The other authors contributed to collection of the data. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
E. Ihara received lecture fees from Takeda Pharmaceutical Co., Ltd., and belongs to an endowed course supported by companies including Ono Pharmaceutical Co., Ltd., Miyarisan Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Fujifilm Medical Co., Ltd., Terumo Corporation, Fancl Corporation, and Ohga Pharmacy. The authors declare no other conflicts of interest in association with this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2020_1725_MOESM1_ESM.tif
Supplementary Figure 1. A pilot study to determine the optimal number of SEL images for the main analysis (Pilot study). The results of the pilot study to determine the optimal number of EUS images required to reliably determine the examinees’ diagnostic ability for the evaluation of SELs are shown. Five EUS experts who did not participate in the main analysis were asked to diagnose the SELs. They were shown five images in one session, and the sessions were repeated eight times using a different set of five images each, resulting in analysis of case numbers ranging from 5 to 40. (TIFF 2234 kb)
Rights and permissions
About this article
Cite this article
Minoda, Y., Ihara, E., Komori, K. et al. Efficacy of endoscopic ultrasound with artificial intelligence for the diagnosis of gastrointestinal stromal tumors. J Gastroenterol 55, 1119–1126 (2020). https://doi.org/10.1007/s00535-020-01725-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-020-01725-4