Abstract
Three major standard treatments, i.e., surgery, chemotherapy, and radiotherapy, were traditionally applied to the treatment of cancer and saved many patients. Meanwhile, clinical studies as well as basic research of immunotherapy are being actively conducted for intractable or advanced malignancies that cannot be cured by the conventional standard treatments. Remarkable therapeutic efficacies have been recently reported in clinical trials on some cancer types, and immunotherapy is now being recognized as the “fourth” standard therapy against cancer. In particular, immune checkpoint inhibitor therapy (ICI) has demonstrated the effectiveness of immunotherapy through large-scale randomized clinical trials, leading to the paradigm-shift in cancer treatment. Immune checkpoint molecules transduce co-inhibitory signals to immunocompetent cells including T cells, and crucially contribute to the formation of an immunosuppressive microenvironment in tumor tissues, which intrinsically confers the treatment resistance. Programmed death-1 (PD-1, CD279) is one of the typical immune checkpoint molecules. Anti-tumor therapies targeting PD-1 and its ligands had been developed and approved in many countries, and various studies utilizing clinical specimens are currently progressing. In this review, we provide an overview of the biomarkers based on the analysis of enteric microbiota that correlate with the clinical efficacy/inefficacy of PD-1-based therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
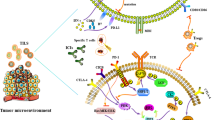
Immune checkpoint molecules regulate the host immune system by transducing immunosuppressive co-signals to immunocompetent cells [1,2,3,4,5]. The most representative ones are CTLA-4 (cytotoxic T-lymphocyte-associated protein 4, CD152) and PD-1/PD-L1 (CD274), PD-L2 (CD273) [6,7,8,9,10,11,12,13]. By being expressed “at appropriate timing” and on “appropriate cell types”, they play a major role in preventing overactivation of host immune system and in keeping immunological homeostasis and tolerance [1, 2, 5]. Meanwhile, it has been reported that immune checkpoint molecules are aberrantly expressed in tumor tissues [3, 14,15,16]. As a result, a potent immunosuppressive milieu is generated in tumor tissues, which is one of the major causes of the treatment resistance in many cancer types. The aim of ICI is to disarm or mitigate the immunosuppressive mechanisms in the tumor microenvironment with inhibitory agents targeting immune checkpoint molecules (Fig. 1) [2, 5, 17]. To date, not only anti-PD-1 antibodies (e.g., nivolumab and pembrolizumab) but also anti-PD-L1 (e.g., atezolizumab, avelumab, and durvalumab) and anti-CTLA-4 (e.g., ipilimumab) antibodies have been approved worldwide as therapeutic medicines for multiple cancer types [18,19,20,21,22,23].
Conceptual diagram of immune checkpoint blockade therapy. a In tumor microenvironment, the immunological balance is conspicuously biased toward inhibitory-dominant side. The aim of cancer immune therapies is to make the balance of the host immunity biased toward stimulatory-dominant side. b In immune checkpoint blockade therapy, the balance is shifted by “decreasing or removing the weights from the inhibitory side” with inhibitory agent against immune checkpoint molecule such as blocking antibodies
Biomarkers associated with clinical efficacy of ICI
Regardless of its clinical success, ICI is facing several problems to be overcome. One of the most crucial issues is identification of biomarkers which correlate with the clinical benefits or with the adverse events [3, 24,25,26]. For example, the response rates of ICI using a single agent against melanoma are about 20–40% [24, 27,28,29,30]. Therefore, establishment of biomarkers enabling appropriate selection of patients is a critical issue from the viewpoint of healthcare cost matter as well as of patients’ quality of life. To date, several biomarkers for predicting the effectiveness or ineffectiveness of ICI have been reported, and many of them rely on cellular or molecular examinations such as immunohistochemistry and flow cytometry. In anti-PD-1 or anti-PD-L1 antibody therapy against several cancer types, some pathological features as follows are correlated with the response rates [3, 18, 20, 31,32,33,34,35,36,37,38]:
-
Infiltration of T cells into or around the tumor tissue.
-
Expression of PD-L1 inside the tumor tissue.
-
Expression of PD-L1 on immune cells infiltrating to the cancer tissues.
Unfortunately, it is claimed that those biomarkers are not universally applicable to many cancer types. Furthermore, many exceptional cases, such as ineffectiveness on PD-L1-positive tumors and effectiveness on PD-L1-negative tumors, are reported, and hence, they cannot perfectly predict the effectiveness or ineffectiveness [24, 39,40,41,42,43]. Therefore, the development of the biomarkers with a new approach has been pursued.
Bacterial biomarkers for PD-1-based therapy against melanoma
The evolution and popularization of next-generation sequencer led to technological innovations in the studies of enteric microbiota, and uncovered that enteric microbiota profoundly affects host immune system [44,45,46,47,48]. This prompted to conduct investigations focusing on the relationship between efficacy of ICI and intestinal flora, and some intriguing findings have been reported mainly in melanoma. The finding that intestinal flora affected the efficacy of a therapy targeting PD-1/PD-L1 axis was initially reported with an experimental murine melanoma model [49]. It was demonstrated that oral administration of Bifidobacterium species including B. breve and B. longum resulted in an improved tumor control without additional treatments, and that the improvement was further augmented in combination with anti-PD-L1 antibody treatment. Tumor-specific T cells increased in the tumor tissue as well as the periphery in those mice, and the depletion of CD8+ T cells canceled the therapeutic effects. Furthermore, the Bifidobacterium feedings enhanced the capacity of dendritic cells to stimulate CD8+ T cells. These suggested that the colonization of Bifidobacterium species modulated dendritic cell activation, leading to the exertion of anti-tumor effects via evoking T cell immunity [49]. Previous studies demonstrated that some species of Bifidobacterium had a potential to modulate DC activation directly and to influence T-cell responses [50,51,52,53,54,55]. Although innate immune systems including Toll-like receptors should be involved [56,57,58,59,60,61], heat inactivation of those bacteria wiped out the anti-tumor effects after their oral administration [49]. This suggested that live bacteria were indispensable and that bacterial components alone were insufficient.
Based on the results described above, analyses using feces of metastatic melanoma patients who had received anti-PD-1 antibody therapies were conducted by the same group at University of Chicago [62]. In that cohort study, stool specimens were collected from 42 metastatic melanoma patients prior to the anti-PD-1 antibody treatment, and the correlations between the compositions of intestinal flora and the therapeutic efficacies were examined. It was demonstrated that eight bacterial species, including Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium, were enriched in the responders to the PD-1-based therapy as compared to the non-responders. On the other hand, two bacterial species, Ruminococcus obeum and Roseburia intestinalis, were found to be more abundant in the non-responders than the responders [62]. Fecal microbiome transplantation into germ-free mice revealed that feces of the responders, but not that of the non-responders, had a capacity to control tumor growth. Fecal transplantation from the responder increased tumor-specific T cells not only in the spleen but also in the tumor tissue of the mice as compared to that from the non-responder, indicating that colonization of the beneficial bacteria primed tumor antigen-specific immunity locally as well as systemically [62]. Furthermore, the experimental fecal transplantation from the responders into mice augmented the therapeutic effects of anti-PD-L1 antibody, whereas that from the non-responders abrogated the effects. Collectively, colonization of several bacterial species including Bifidobacterium longum, which was found also in the murine study described above, was associated with anti-tumor efficacies of PD-1-based therapy in the cohort of this study [62].
In another cohort study conducted at The University of Texas MD Anderson Cancer Center, fecal samples were collected from 112 metastatic melanoma patients before and after the anti-PD-1 antibody treatment, and the correlations between the diversity and compositions of the intestinal flora and the clinical responses were analyzed [63]. The diversity of microbiota of the responders of the therapy was profoundly higher than that of the non-responders, resulting that the patients with high diversity had significantly longer progression-free survival (PFS) than those with intermediate or low diversity [63]. It was also uncovered that Ruminococcaceae family and Faecalibacterium genus were enriched in fecal microbiota of the responders, whereas Bacteroidales was abundant in those of the non-responders. Consistent with this finding, patients with high Faecalibacterium abundance displayed longer PFS than those with lower abundance, while patients with high Bacteroidales abundance had shorter PFS than those with lower abundance [63]. Immunohistochemical analyses of the tumor tissues revealed that the infiltration of CD8+ T cells into the tumor and the abundance of the Faecalibacterium, the Ruminococcaceae, and the Clostridiales in the gut were positively correlated [63]. Moreover, in the systemic circulation, the patients with the high abundance of the Faecalibacterium, the Ruminococcaceae, and the Clostridiales displayed high frequencies of CD8+ T cells and effector CD4+ T cells. In contrast, the patients with the high abundance of the Bacteroidales exhibited high frequencies of immune-suppressive cell populations such as regulatory T cells (Treg) and myeloid-derived suppressor cells in the systemic circulation [63]. Fecal microbiome transplantation into germ-free mice revealed that feces of the responders significantly suppressed tumor growth as compared to that of the non-responders. Moreover, the experimental fecal transplantation from the responders into mice improved the therapeutic efficacy of anti-PD-L1 antibody treatment, although that from the non-responders worsened [63]. Tumor tissues of the mice receiving the feces from the responders exhibited higher levels of CD8+ T cell infiltration and of PD-L1 expression than those form the non-responders, suggesting that colonization of the beneficial bacteria in the gut would generate the immunologically “hot” microenvironment in the tumor tissues. Moreover, high frequency of innate immune effector cells expressing CD45, CD11b, and Ly6G [64], and low frequency of myeloid suppressor cells expressing CD11b and CD11c [65] were observed in the tumor tissues of mice receiving the feces of the responders as compared to those of non-responders. In contrast, the mice receiving the feces of the non-responders displayed higher frequencies of Treg in their spleens than those of the responders [63]. Consistent with the aforementioned results in another cohort, these results indicated that colonization of specific bacteria in the gut would influence anti-tumor immunity not only systemically but also locally. Altogether, in PD-1-based therapy on the cohort of this study, enrichment of Ruminococcaceae family and Faecalibacterium genus was correlated with the effectiveness, whereas that of Bacteroidales was correlated with the ineffectiveness [63].
Bacterial biomarkers for PD-1-based therapy against cancers other than melanoma
In the cohort study conducted at three clinical sites in France, the correlation between antibiotic treatment and the efficacy of PD-1-based therapy was investigated on 249 patients with epithelial cancers including non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC) and urothelial carcinoma [66]. The patients who were treated with antibiotics before or after the antibody therapy displayed shortened PFS and overall survival as compared with those who were not treated with antibiotics, and this was also the case in experimental murine models. These suggested that dysbiosis might affect the efficacies of anti-PD-1/PD-L1 antibody therapy [66]. Based on those results, fecal microbiome analyses were conducted on the NSCLC and RCC patients. By comparing between the responders and non-responders of the PD-1-based therapy, the intestinal bacterium most significantly associated with beneficial therapeutic responses in both NSCLC and RCC patients was Akkermansia muciniphila [66]. Interestingly, the duration of PFS was positively correlated with the IFN-γ production from peripheral blood CD4+ T cells and CD8+ T cells in response to A. muciniphila but not to TCR ligation. This might indicate that T cell responses specific to A. muciniphila, but not non-specific bystander responses, had some relationships with anti-tumor effects. Fecal microbiome transplantation from the responders of the PD-1-based therapy into antibiotic-treated or germ-free mice restored the anti-tumor efficacy of anti-PD-1 antibody treatment although that from the non-responders did not [66]. Upon the fecal transplantation from the responders, the accumulation of CXCR3+ CD4+ T cells, which is a characteristic feature of Th1 [67], in the tumor tissues as well as the up-regulation of PD-L1 on CD4+ T cells in the spleens were observed. Mono-colonization with A. muciniphila on the tumor-bearing mice treated with antibiotics restored the sensitivity to anti-PD-1 antibody treatment. Furthermore, oral administration of A. muciniphila into the mice that received the fecal transplantation from the non-responders ameliorated the efficacy of the anti-PD-1-based therapy. Immunohistochemical examination exhibited that in the tumor tissues of mice co-treated with A. muciniphila and anti-PD-1 antibody, but not in those treated with the antibody alone, the ratio of CD4 to FoxP3, a definitive transcription factor for Treg [68], was increased, suggesting that the immune-stimulatory condition was induced in the tumor after the combinatory treatment. Furthermore, A. muciniphila stimulated dendritic cells in vitro to produce IL-12, which is the crucial cytokine for the differentiation to Th1 [69]. The neutralization of IL-12 or IFN-γ with the specific antibodies eliminated the in vivo anti-tumor efficacy by the co-treatment of anti-PD-1 antibody and A. muciniphila. Taken together, colonization of A. muciniphila would play an important role in the efficacy of the therapy targeting PD-1/PD-L1 axis against some types of cancers through the induction of Th1 responses [66].
Future perspectives of biomarkers
At present, a large number of clinical trials of ICI are ongoing worldwide, and analyses using clinical specimens including feces are also actively being conducted. In this review, we focused on enteric microbiota that could be biomarkers correlating with the efficacy/inefficacy of PD-1-based therapies. Interestingly, it has also been reported that the efficacy of CTLA-4 blockade therapy against melanoma is associated with some enteric bacteria, Bacteroides fragilis and/or B. thetaiotaomicron [70]. Nonetheless, it is unlikely that those bacteria alone can be the complete biomarker universally applicable to many cancer types. Besides the intestinal bacteria described here, several biomarkers predicting clinical benefits or adverse events based on genetic analysis have been reported so far, listed as follows:
-
Tumor cells
-
1.
Mutation burden leading to the generation of neoantigens [71, 72].
-
2.
Activation of Wnt/β catenin signals [73].
-
3.
Loss-of-function mutation of the genes related to DNA mismatch repair system [74, 75].
-
4.
3′-UTR disruption of PD-L1 gene leading to the aberrant expression of PD-L1 [76].
-
Other than tumor cells
- 1.
- 2.
Henceforth, it is anticipated that more accurate prediction of clinical benefits and/or adverse events will be realized by comprehensive integration of multiparametric biomarkers for individual patients, and that artificial intelligence would play a crucial role in the selection of optimal patients or therapy (Fig. 2).
Future perspectives of biomarker for immune checkpoint blockade therapy. It is expected that more accurate prediction of clinical benefits and/or adverse events will be realized by comprehensive integration of multiparametric biomarkers for individual patients, containing histological and cytometric examination and genetic analysis of tumor cell and non-tumor cells. In that process, artificial intelligence would play a crucial role in the scoring procedure for the selection of optimal patients or therapy
At present, the precise molecular mechanisms by which some specific bacteria described above affect the anti-tumor efficacy remain unsolved [81, 82]. For example,
-
the speciality of those bacteria: do they have special component(s) and/or secreted material(s) that do not exist in other bacteria?
-
the specificity to tumor: do they have T cell epitope(s) resembling tumor-associated antigen(s) and/or neoantigen(s), and induce the cross-reaction to tumor?
-
the difference in bacterial species among the cohorts: does the difference in the basal intestinal microbiota due to ethnic groups, dietary habits, living environments, and so on, affect the difference of those beneficial bacteria?
-
the “remote control” of anti-tumor immunity: how does the colonization of those bacteria in intestinal tract modulate the efficacies of ICI at distal tumor sites?
By elucidating the issues, some enteric bacteria or their derivatives, i.e., probiotics, can be candidate agents for the novel combination therapy utilizing immune checkpoint inhibitors [82, 83].
References
Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42.
Adachi K, Tamada K. Immune checkpoint blockade opens an avenue of cancer immunotherapy with a potent clinical efficacy. Cancer Sci. 2015;106:945–50.
Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82.
Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19:4917–24.
Baumeister SH, Freeman GJ, Dranoff G, et al. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–73.
Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95.
Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51.
Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science (New York, NY). 2001;291:319–22.
Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9.
Tamura H, Dong H, Zhu G, et al. B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood. 2001;97:1809–16.
Dong H, Zhu G, Tamada K, et al. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–36.
Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8.
Wang S, Bajorath J, Flies DB, et al. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083–91.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science (New York, NY). 1996;271:1734–6.
Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800.
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (New York, NY). 2018;359:1350–5.
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44.
Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62.
Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–85.
Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34:3119–25.
Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Meng X, Huang Z, Teng F, et al. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41:868–76.
Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87.
Gnjatic S, Bronte V, Brunet LR, et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J Immunother Cancer. 2017;5:44.
Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8.
Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–9.
Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: pD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764–82.
Franklin C, Livingstone E, Roesch A, et al. Immunotherapy in melanoma: recent advances and future directions. Eur J Surg Oncol. 2017;43:604–11.
Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7.
Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39.
Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84.
Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13.
Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71.
Vassilakopoulou M, Avgeris M, Velcheti V, et al. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. 2016;22:704–13.
Vilain RE, Menzies AM, Wilmott JS, et al. Dynamic changes in pd-l1 expression and immune infiltrates early during treatment predict response to PD-1 blockade in melanoma. Clini Cancer Res. 2017;23:5024–33.
Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–56.
Chatterjee M, Turner DC, Felip E, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol. 2016;27:1291–8.
Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–26.
Teixido C, Vilarino N, Reyes R, et al. PD-L1 expression testing in non-small cell lung cancer. Ther Adv Med Oncol. 2018;10:1758835918763493.
Zhu J, Armstrong AJ, Friedlander TW, et al. Biomarkers of immunotherapy in urothelial and renal cell carcinoma: PD-L1, tumor mutational burden, and beyond. J Immunother Cancer. 2018;6:4.
Atarashi K, Umesaki Y, Honda K. Microbiotal influence on T cell subset development. Semin Immunol. 2011;23:146–53.
McAleer JP, Kolls JK. Maintaining poise: commensal microbiota calibrate interferon responses. Immunity. 2012;37:10–2.
Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science (New York, NY). 2012;336:1268–73.
Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6.
Ohnmacht C, Park JH, Cording S, et al. Mucosal immunology. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science (New York, NY). 2015;349:989–93.
Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science (New York, NY). 2015;350:1084–9.
Lopez P, Gueimonde M, Margolles A, et al. Distinct Bifidobacterium strains drive different immune responses in vitro. Int J Food Microbiol. 2010;138:157–65.
Baba N, Samson S, Bourdet-Sicard R, et al. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J Leukoc Biol. 2008;84:468–76.
Latvala S, Pietila TE, Veckman V, et al. Potentially probiotic bacteria induce efficient maturation but differential cytokine production in human monocyte-derived dendritic cells. World J Gastroenterol. 2008;14:5570–83 discussion 81–2.
Medina M, Izquierdo E, Ennahar S, et al. Differential immunomodulatory properties of Bifidobacterium longum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol. 2007;150:531–8.
Hart AL, Lammers K, Brigidi P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–9.
Young SL, Simon MA, Baird MA, et al. Bifidobacterial species differentially affect expression of cell surface markers and cytokines of dendritic cells harvested from cord blood. Clin Diagn Lab Immunol. 2004;11:686–90.
Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–50.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
Kawai T, Akira S. Pathogen recognition with toll-like receptors. Curr Opin Immunol. 2005;17:338–44.
Pasare C, Medzhitov R. Toll-like receptors and acquired immunity. Semin Immunol. 2004;16:23–6.
Barton GM, Medzhitov R. Control of adaptive immune responses by toll-like receptors. Curr Opin Immunol. 2002;14:380–3.
Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science (New York, NY). 2001;293:253–6.
Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science (New York, NY). 2018;359:104–8.
Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (New York, NY). 2018;359:97–103.
Liu Y, O’Leary CE, Wang LS, et al. CD11b + Ly6G + cells inhibit tumor growth by suppressing IL-17 production at early stages of tumorigenesis. Oncoimmunology. 2016;5:e1061175.
Norian LA, Rodriguez PC, O’Mara LA, et al. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via l-arginine metabolism. Can Res. 2009;69:3086–94.
Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (New York, NY). 2018;359:91–7.
Ward SG, Bacon K, Westwick J. Chemokines and T lymphocytes: more than an attraction. Immunity. 1998;9:1–11.
Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87.
Magram J, Connaughton SE, Warrier RR, et al. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–81.
Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (New York, NY). 2015;350:1079–84.
McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (New York, NY). 2016;351:1463–9.
Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (New York, NY). 2015;348:69–74.
Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–5.
Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (New York, NY). 2017;357:409–13.
Kataoka K, Shiraishi Y, Takeda Y, et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature. 2016;534:402–6.
Robert L, Tsoi J, Wang X, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res. 2014;20:2424–32.
Cha E, Klinger M, Hou Y, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med. 2014;6:238ra70.
Riaz N, Havel JJ, Makarov V, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell. 2017;171(934–49):e15.
Roh W, Chen PL, Reuben A, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. 2017;9:eaah3560.
Buchta Rosean CM, Rutkowski MR. The influence of the commensal microbiota on distal tumor-promoting inflammation. Semin Immunol. 2017;32:62–73.
Zitvogel L, Ma Y, Raoult D, et al. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science (New York, NY). 2018;359:1366–70.
Marinelli L, Tenore GC, Novellino E. Probiotic species in the modulation of the anticancer immune response. Semin Cancer Biol. 2017;46:182–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Adachi, K., Tamada, K. Microbial biomarkers for immune checkpoint blockade therapy against cancer. J Gastroenterol 53, 999–1005 (2018). https://doi.org/10.1007/s00535-018-1492-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-018-1492-9