Abstract
Organoid technologies to expand intestinal epithelial cells are gaining increasing attention as a useful tool to investigate many aspects of intestinal epithelial biology and pathology. One important application of organoid systems would be to use intestinal epithelial cells expanded in culture for following transplantation experiments. In this article, we present a brief overview of the studies that have succeeded in generating new epithelial tissues in the surface of native intestines in mice by organoid transplantation. We also discuss possible applications of this experimental approach in basic research on the intestinal epithelium as well as in regenerative medicine for various types of intestinal diseases in humans.
Similar content being viewed by others
Introduction
In 2009, Sato, Clevers, and colleagues published a seminal paper that revolutionized research on the intestinal epithelium. They demonstrated a long-awaited technological breakthrough in culturing normal, untransformed intestinal epithelial cells, expanding their stem cell populations ex vivo [1]. Following this, studies from the same lab and others, including ours, further showed the methodologies to culture various types of intestinal epithelial cells such as small intestinal cells, colonic cells, and even fetal intestinal epithelial progenitors of both mouse and human as three-dimensional structures called organoids [2–7]. Intestinal epithelia grown in these 3D organoid systems recapitulate many aspects of their structure and function in in vivo settings. Thus, these organoid systems are gaining much attention as the organotypic model to study tissue homeostasis, developmental processes, host–microbe interactions, and the mechanisms of the diseases in the intestine [8–12].
One of the multifaceted applications of organoid systems would be to use intestinal epithelial cells expanded in culture for subsequent transplantation experiments. Such experimental approaches would provide us with unprecedented opportunities to investigate how different types of intestinal epithelial cells behave when they are reintroduced to the body, after their purification, expansion, or manipulation ex vivo. Based on this notion, we assessed whether mouse colonic organoid cells are able to engraft in vivo and then demonstrated the capability of those cultured cells to regenerate colonic epithelial tissues when transplanted [4]. This result not only opened up the possible use of organoid transplantation experiments for basic research but also established the feasibility of stem cell transplantation to repair injured intestines in human diseases. In this article, we present a short overview of the studies that leverage the combination of organoid culture systems and transplantation methodologies of intestinal epithelial cells. We also discuss future directions of research in this field, which could be pursued by this particular experimental approach.
Intestinal organoids
Researchers in the field of intestinal epithelial biology struggled for a long time to culture intestinal epithelial cells, aiming to work with those cells for a variety of purposes on a clean bench in a laboratory [13, 14]. However, despite extensive efforts, long-term intestinal cell culture remained challenging. In 2009, Sato et al. overcame this challenge by developing a protocol for the isolation and culture procedures of primary intestinal epithelial cells [1]. They showed that when crypt cells of the mouse small intestine are placed three-dimensionally in a basement membrane-derived extracellular matrix (Matrigel), the cells grow for a long period of time in the presence of epidermal growth factor (EGF), noggin, and R-spondin in the culture medium, even in the absence of non-epithelial cells. Under this condition, small intestinal crypts form unique 3D structures that are composed of a central spherical domain as well as many budding structures that protrude outward from the center. As the cells expressing Lgr5, a marker gene for intestinal epithelial stem cells, and Paneth cells both reside at the apex of budding structures, these outer parts are thought to represent the crypt structure of the small intestinal epithelium. The central part of the structure is considered to be equivalent to the villus structure, as it is composed mostly of differentiated cells. Following this seminal work, several reports, including ours, showed that colonic epithelial cells derived from mice and humans are also able to grow in culture without any aid from non-epithelial cell types [2–4]. Although several differences in methodologies were noted in those reports, the most remarkable difference between the methods for small intestinal and colonic cell culture was the requirement for Wnt ligands in the culture medium for the latter. The rationale behind this different property in these two epithelial cell types was provided only a short while later. Sato et al. demonstrated that since Paneth cells, which are present only in the small intestinal crypt, serve as the local source of Wnt factors in culture, small intestinal stem cells are able to grow in the absence of Wnt supplementation [15]. By contrast, as the colonic epithelium does not contain Paneth-like cells that produce sufficient amount of Wnts, it requires exogenous Wnt addition in culture for growth. Several studies have suggested the existence of a particular type of colonic cells, which are located at crypt bases and shown to have some supportive functions for stem cell populations [16, 17]. However, whether or not these cells are the functional equivalent of Paneth cells requires further investigation.
In addition to the organoid system for small intestinal and colonic cells, a system for fetal intestinal progenitor cells was also established. The developmental process of the intestinal epithelium is dynamic but long-lasting, and the tissue remains in an immature state even at birth. Fordham et al. developed a method to grow three-dimensionally the epithelial progenitor cells obtained from mice and human in fetal stages [6]. They demonstrated that, for example, the intestinal cells obtained from mice in late fetal stages can be maintained as spherical structures called fetal enterospheres (FEnS). These cells were shown to grow in the absence of R-spondin or even in the presence of Wnt inhibitors, which indicates their distinctive features from intestinal organoids derived from adult mice. Interestingly, the study showed that the cells constituting FEnS are able to mature to form adult-type organoids when the Wnt signal is activated. Such maturation potential of these cells suggests that FEnS may represent a population of cells that phenocopy the characteristics of fetal intestinal cells capable of maturing into adult-type epithelia during the perinatal period of development.
We do not describe the experimental details of the organoid and FEnS culture systems in this review, as they have been discussed in recent reviews [18, 19]. However, what we should emphasize is that the organotypic culture systems for various types of intestinal cells are increasingly becoming an important tool for multiple disciplines in intestinal epithelial research [8–12].
Early work of intestinal epithelial cell transplantation
Lgr5+ cells are able to form organoids containing all types of terminally differentiated intestinal epithelial cells, even when they are seeded at a density of one cell per well in culture dishes [1]. In addition, the procedure can be repeated multiple times when the organoids are dissociated into single cells and reseeded in dishes [1]. This clearly indicates that, based at least on their in vitro behaviors, the Lgr5+ cells meet the basic requirements of stem cells: self-renewal capacity and multi-differentiation potential. However, it remained unclear whether they represent genuine stem cell populations that are capable of self-renewing and regenerating the functional epithelia in vivo. Such strong proof of stemness inherent in a particular cell type can be provided when those cells are shown to repopulate the tissues in in vivo models of transplantation, retaining their ability to self-renew and differentiate into multiple lineages through a long period of time.
Even before the emergence of organoid technology, researchers made attempts to graft intestinal epithelial cells in vivo. Early studies used endodermal cells in developmental stages as a source of transplantation. For instance, Kedinger et al. grafted fetal rat endoderm cells along with fetal rat gut mesenchyme under the kidney capsule of adult rats [20]. They showed that graft cells formed well-vascularized mucosal structures that contained multiple types of terminally differentiated intestinal epithelial cells. Other studies showed that rat fetal endoderm cells were able to develop intestinal mucosa that contained differentiated cells when xenografted under the skin of nude mice [21, 22]. In these experiments, the graft tissues were analyzed by 4 weeks post-transplantation at the latest, which seemed insufficient to demonstrate long-term self-renewal of stem cells in the graft. In addition, identification of intestinal stem cells with their specific molecular markers or in vivo genetic lineage tracing approaches remained unavailable at the time [23, 24]. Thus, these early studies of transplantation could not yet provide proof of the presence of functional stem cells in the graft tissues.
Despite the lack of methodologies to identify and locate intestinal stem cells formally in graft tissues, researchers in the field worked steadily to transplant intestinal stem cells even into the surface of native intestines. Tait et al. described a rat model in which they created a freely isolated loop of the ascending colon that kept blood supply intact in the abdomen. By applying surgical mucosectomy to this intestinal segment, they used this anatomical site for transplantation of epithelial cells that were isolated from the small intestine of postnatal rats [25]. Two weeks after seeding the suspension of donor-derived cells over the surface of the colonic loop, they found that the transplants were able to survive ectopically, regenerating the tissue that exhibited the small intestinal phenotype [25]. Stelzner and his group succeeded in orthotopically grafting the epithelial cells that were isolated from the small intestine [26]. They isolated a part of the jejunum with its blood circulation unaffected, then stripped away its epithelial component by exposing the luminal surface to a chelating agent, ethylene diamine tetraacetic acid (EDTA). By using this isolated small intestine as a site of transplantation, they seeded small intestinal epithelial cells isolated from neonatal mice and then analyzed the tissues 3 weeks later. It was demonstrated that transplanted cells generated neomucosa, which showed typical small bowel morphology that was indistinguishable from the adjacent native mucosa. The group further extended their study not only to develop a rat model of orthotopic transplantation of the small intestinal epithelium, but also to show that the cells obtained from a particular segment of the small intestine, the ileum, could engraft the jejunum, preserving the expression of the Slc10A2 gene (also known as the Ibat or the Asbt) that encodes an ileal bile acid transporter [27]. Furthermore, the group demonstrated that this strategy to replace a part of the jejunal epithelium with the ileal one could lead to the generation of functional ileal epithelia within the jejunum [28]. They showed that when they transplanted ileal epithelial cells into the jejunum as described above, and then anastomosed the freely isolated jejunum in its original place to restore the continuity of the bowel, this “neoileum” was functional in regard to bile acid absorption and corrected a clinical manifestation of malabsorption [28]. Again, as these studies were done before the availability of specific molecular markers or in vivo genetic lineage tracing approaches to document stem cell populations in the graft, whether or not graft cells contained functional stem cells remained unanswered. However, those studies had several important implications. They demonstrated that the epithelial cells of a part of the intestine could be stripped in living animals, leaving the denuded surface of the intestine usable as a scaffold for cell transplantation. Furthermore, it was clearly shown that at least a certain number of intestinal cells could survive during the procedures of isolation, processing for transplantation, and the following period of several weeks in the graft tissues of recipients.
Transplantation of stem cells grown in colonic organoids
On the basis of these early studies, and also fueled by the advent of organoid technology, we sought to develop a mouse model of cell transplantation to assess tissue regeneration capabilities of intestinal epithelial cells expanded in organoids [4]. We used an acute colonic injury model as a recipient since the anatomy of the colon allowed for a simple and unique access to deliver the cultured cells by means of an enema. When colonic mucosal damage was induced by providing immunocompromised Rag2–/– mice with colitis-inducing dextran sulfate sodium (DSS), these recipient mice developed colitis characterized by weight loss, bloody stool, and diarrhea, due to epithelial injuries in the most distal area of the colon [29]. Meanwhile, colonic epithelial cells from EGFP transgenic mice [30] were expanded as organoids and used as donor cells. The EGFP+ colonic organoids were dissociated into small fragments, suspended in a Matrigel-containing PBS, and then infused into the colonic lumen of recipient mice.
Interestingly, shortly after the transplantation when the recipient colon showed varying degrees of tissue recovery, multiple EGFP+ areas were found as discrete areas in those tissues. Histologically, EGFP+ cells covered the denuded submucosa and lay between less damaged recipient tissues. Importantly, mice that underwent transplantation and had successful engraftment regained their body weight faster than those of sham-transplanted mice. At 4 weeks post-transplantation, the EGFP+ cells were shown to form the typical structure of colonic crypts that were indistinguishable from surrounding EGFP− epithelium. They contained actively proliferating cells and all terminally differentiated cell types (i.e., goblet cells, enteroendocrine cells, mature colonocytes, and tuft cells) [4].
Upon seeing this successful result, we then sought to initiate the entire organoid expansion/transplantation protocol from a single stem cell. In collaboration with Clevers Lab, we crossed Lgr5-EGFP-Ires-CreERT2 mice with R26R-Confetti reporter mice [31], and grew the colonic cells of their offspring. In those offspring mice, tamoxifen-induced Cre activation resulted in stochastically-selected expression of one out of four fluorescent proteins (RFP, CFP, GFP, and YFP) in Lgr5+ stem cells, which allowed us to track and visualize single stem cell-derived progeny by fluorescent signals. Three days after Cre activation in mice, we isolated colonic crypts, dissociated them into single cells, and sorted the cells that were double-positive for EGFP (derived from the Lgr5 knock-in allele) and RFP (expressed from the R26R-Confetti allele as a result of random recombination). The sorted stem cells were cultured from a single cell, exponentially expanded, and then transplanted into multiple mice that were treated with DSS in advance. We found successful engraftment of RFP+ cells in multiple mice that were analyzed at different time points, including one maintained for a prolonged time period of 6 months post-transplantation. On histology, RFP+ tissues were found to be composed of colonic crypts that contained all differentiated cell types and Ki67+ proliferating cells. These data clearly indicate that the cells retaining their stem cell properties are able to increase in number by serial passages of organoid culture from a single stem cell, and then function to regenerate multiple new colonic crypts in multiple mice after being transplanted [4].
Transplantation of small intestinal stem cells expanded in culture
Following this successful transplantation of colonic stem cells, we were interested in whether the organoid cells derived from the small intestine were also transplantable. To address this, we collaborated with Jensen Lab to perform transplantation of fetal small intestinal cells grown as FEnS [6]. We found that when FEnS cells were transplanted following the same protocol used for colonic cell transplantation, those fetal cells were able to generate new epithelia in distal colonic epithelia in adult mice. Interestingly, the FEnS-derived grafts expressed CA2, a protein known to be present only in the colonic epithelium. In addition, the graft tissues did not express marker proteins normally detectable in the small intestine such as Lysozyme and alkaline-phosphatases. This suggests that the small intestinal cells in fetal stages have plasticity in terms of their differentiation potentials, and then acquire colonic phenotypes when they are heterotopically transplanted [6].
By taking similar approach, we next assessed how cultured small intestinal cells of adult origin behave when they are placed in the colonic environment. For this study, we developed a new model of recipient mice in order to obtain large areas of colonic epithelial injury that potentially allow engraftment of transplanted cells [32]. By providing topical exposure of colonic lumen to an EDTA solution and following mechanical abrasion, we generated superficial damage in confined areas of the distal colon within a short period of time. When we performed transplantation of adult small intestinal organoid cells, those transplanted cells successfully repopulated those areas of immunocompetent, wild-type recipients. Newly generated epithelia were composed of all differentiated cell types of the small intestine, but not of the colonic cells such as CA2+ cells. In addition, within some areas of the EGFP+ grafts, there emerged structures that were reminiscent of the typical villus structure of the small intestine. This showed that, even in a heterotopic environment, graft cells retained their original features in regard to cellular component and their morphological characteristics of small intestinal phenotype. Lysozyme-positive Paneth cells were present exclusively at the crypt bottoms of graft tissues. Moreover, OLFM4, which is known to label stem cell populations of the small intestine, but not of the colon [33, 34], was also expressed exclusively in the graft epithelium. All these features of the graft tissue were noted even at 4 months post-transplantation. These results demonstrate that cultured adult small intestinal cells constitute the small intestinal-type stem cell compartment at crypt bases, and then generate new epithelia that morphologically and functionally preserve the small intestinal phenotype in the colonic environment. This study proposed the presence of an epithelium-intrinsic program that allows adult small intestinal stem cells to maintain their region-specific identity along the intestinal tract [32]. In addition, along with the results of fetal cell transplantation, the study suggested that such an epithelium-intrinsic mechanism is acquired sometime after late fetal stages.
Applicable use of organoid cell transplantation in future research
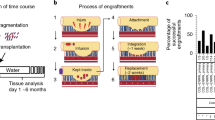
Transplanting organoid cells, particularly following their in vitro manipulation and expansion, will provide unique strategies to investigate many important aspects of intestinal epithelial biology and pathology in an unprecedented way (Fig. 1). At the present time, in order to assess the function of the gene of interest in the intestinal epithelium, combinatorial technology of conditional gene manipulation and functional induction of various recombinase proteins is widely employed [35, 36]. Such tissue-specific gene manipulation techniques, however, basically depend on the endogenous promoter activity of the gene chosen for the system, and thus yield broad areas of mutated epithelium in an uninterrupted fashion. By contrast, the organoid transplantation is able to produce well-demarcated areas of graft tissues in a patchy distribution pattern on the continuing intestinal surface in recipients. This may allow us to investigate how two different types of epithelial cells compete when they are placed next to each other and identify the mechanisms of these local competitions between neighboring cells. If we transplant cells engineered to have immune or barrier abnormalities [37, 38], it may allow us to study the mechanisms of initiation and promotion of local inflammation and its spreading process over the planar epithelial surface.
Transplantation of organoid cells will also be of help to understand the underlying mechanisms of epithelial–mesenchymal interactions in in vivo settings. Appropriate organization of the intestinal epithelium during development as well as in the adult organ is thought to be controlled by elaborate interactions between the epithelial and mesenchymal cells, involving permissive and instructive signals [39–41]. For example, a permissive signal from the mesenchymal cells allows the epithelium to progress to its pre-assigned fate. By contrast, an instructive signal from the mesenchyme induces differentiation of naïve epithelia toward a particular fate. Grafting assays of various cells derived from different developmental stages, or different portions along the entire intestinal tract will facilitate direct assessment of these interactions within the context of physiological conditions. Recent studies by the groups led by Collins and Daley indeed showcased the usefulness of this approach. They developed a novel platform named CellNet, which allows for computational comparison of the gene regulatory networks (GRNs) of cells manufactured in vitro with those of their in vivo counterparts [42]. By applying CellNet to analyze hepatocyte-like cells (iHeps) that were generated by transcription factor-driven conversion from fibroblasts [43], they found that the GRN status in iHeps is less similar to that in differentiated hepatocytes than to that in endodermal progenitors, which retain the potential for intestinal cell lineage specification [44]. The authors then directly tested the potential for iHeps to differentiate toward intestinal cells in vivo by transplanting iHeps into damaged mouse colons by using the DSS colitis model, and showed that engrafted iHeps were able to acquire the colon GRN and regenerate colonic epithelia in recipients [44]. These data clearly demonstrated that the CellNet system is able to assess accurately the identity of cells engineered in vitro. In addition, the results of transplantation experiments that they described and those we performed with small intestinal cells [6, 32] collectively indicate that the mesenchymal component of the adult colon is able to provide some instructive signals to direct the fate of immature cells, such as engineered endoderm progenitor cells and fetal intestinal cells, toward the colonic phenotype.
The successful engraftment of colonic organoid cells or iHeps cells into a DSS-colitis model and their beneficial effects on clinical outcome of colitis [4, 44] have also led to an idea that organoid transplantation could be a novel approach to treat human intestinal diseases. Of course we need to validate whether we are able to obtain clinical grade human organoid cells suitable for use in therapeutic applications. Such endeavors may include refinement of methods to replace all factors required for culture with GMP grade reagents and the development of systems to assess contamination of infectious pathogens or the risk of tumorigenicity in human organoid cells. However, if it becomes possible to grow various types of organoid cells safely and efficiently, transplantation might be an attractive option to treat human diseases that are characterized by severe intestinal epithelial damage, such as ulcerative colitis, in combination with currently available therapies. The results obtained from transplantation experiments of adult small intestinal organoids in mice may also have implications for regenerative medicine. Short bowel syndrome (SBS) is a disorder caused by surgical resection of the small intestine, which leads to a state of malnutrition because of reduced absorptive capacity of the residual bowel. Patients with severe SBS require long-term parenteral nutrition or intestinal transplantation when the absorptive capacity of the remnant bowel is insufficient to meet the body’s needs for nutrients. If adult small intestine-derived cells are able to retain all their absorptive functions over a long period of time even in the colonic milieu, it will develop the concept of novel tissue engineering that allows a part of colonic tissues to compensate for functions of the small intestine by heterotopic transplantation of adult small intestinal stem cells expanded in culture.
Conclusions
Recent developments in organoid technology to maintain and expand intestinal epithelial cells have impacted a variety of research in intestinal epithelial biology. We have reviewed the studies in which those organoid cells are used for in vivo transplantation assays. Such an experimental approach will become a unique and unprecedented tool for intestinal research. It will also build a basis for developing a novel strategy to repair injured intestinal mucosa or to compensate for the loss of intestinal function in various types of diseases in humans.
References
Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5.
Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–72.
Jung P, Sato T, Merlos-Suárez A, et al. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–7.
Yui S, Nakamura T, Sato T, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618–23.
Wang F, Scoville D, He XC, et al. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology. 2013;145:383–395.
Fordham RP, Yui S, Hannan NR, et al. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell. 2013;13:734–44.
Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc. 2013;8:2471–82.
Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–4.
Kretzschmar K, Clevers H. Organoids: modeling development and the stem cell niche in a dish. Dev Cell. 2016;38:590–600.
Bartfeld S. Modeling infectious diseases and host-microbe interactions in gastrointestinal organoids. Dev Biol. 2016. doi:10.1016/j.ydbio.2016.09.014.
Schweiger PJ, Jensen KB. Modeling human disease using organotypic cultures. Curr Opin Cell Biol. 2016;43:22–9.
Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–97.
Evans GS, Flint N, Somers AS, et al. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J Cell Sci. 1992;101(Pt 1):219–31.
Fukamachi H. Proliferation and differentiation of fetal rat intestinal epithelial cells in primary serum-free culture. J Cell Sci. 1992;103(Pt 2):511–9.
Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–8.
Rothenberg ME, Nusse Y, Kalisky T, et al. Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology. 2012;142(1195–1205):e1196.
Sasaki N, Sachs N, Wiebrands K, et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci USA. 2016;113:5399–407.
Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–54.
Date S, Sato T. Mini-gut organoids: reconstitution of the stem cell niche. Annu Rev Cell Dev Biol. 2015;31:269–89.
Kedinger M, Simon-Assmann PM, Lacroix B, et al. Fetal gut mesenchyme induces differentiation of cultured intestinal endodermal and crypt cells. Dev Biol. 1986;113:474–83.
Del Buono R, Fleming KA, Morey AL, et al. A nude mouse xenograft model of fetal intestine development and differentiation. Development. 1992;114:67–73.
Duluc I, Freund JN, Leberquier C, et al. Fetal endoderm primarily holds the temporal and positional information required for mammalian intestinal development. J Cell Biol. 1994;126:211–21.
Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33.
Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–84.
Tait IS, Evans GS, Flint N, et al. Colonic mucosal replacement by syngeneic small intestinal stem cell transplantation. Am J Surg. 1994;167:67–72.
Avansino JR, Chen DC, Woolman JD, et al. Engraftment of mucosal stem cells into murine jejunum is dependent on optimal dose of cells. J Surg Res. 2006;132:74–9.
Avansino JR, Chen DC, Hoagland VD, et al. Orthotopic transplantation of intestinal mucosal organoids in rodents. Surgery. 2006;140:423–34.
Avansino JR, Chen DC, Hoagland VD, et al. Treatment of bile acid malabsorption using ileal stem cell transplantation. J Am Coll Surg. 2005;201:710–20.
Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–6.
Okabe M, Ikawa M, Kominami K, et al. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–9.
Snippert HJ, van der Flier LG, Sato T, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–44.
Fukuda M, Mizutani T, Mochizuki W, et al. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014;28:1752–7.
van der Flier LG, Haegebarth A, Stange DE, et al. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–7.
van der Flier LG, van Gijn ME, Hatzis P, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–12.
Robine S, Jaisser F, Louvard D. Epithelial cell growth and differentiation. IV. controlled spatiotemporal expression of transgenes: new tools to study normal and pathological states. Am J Physiol. 1997;273:G759–62.
Gierut JJ, Jacks TE, Haigis KM. Strategies to achieve conditional gene mutation in mice. Cold Spring Harb Protoc. 2014;2014:339–49.
Kagnoff MF. The intestinal epithelium is an integral component of a communications network. J Clin Invest. 2014;124:2841–3.
Vereecke L, Beyaert R, van Loo G. Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol Med. 2011;17:584–93.
Kedinger M, Duluc I, Fritsch C, et al. Intestinal epithelial-mesenchymal cell interactions. Ann N Y Acad Sci. 1998;859:1–17.
McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology. 2009;136:2074–91.
Spence JR, Lauf R, Shroyer NF. Vertebrate intestinal endoderm development. Dev Dyn. 2011;240:501–20.
Cahan P, Li H, Morris SA, et al. Cell Net: network biology applied to stem cell engineering. Cell. 2014;158:903–15.
Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–3.
Morris SA, Cahan P, Li H, et al. Dissecting engineered cell types and enhancing cell fate conversion via Cell Net. Cell. 2014;158:889–902.
Acknowledgements
We thank Lauren Unik for manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Part of this review was presented at The 5th International Forum of the 102nd General Meeting of the Japanese Society of Gastroenterology.
Rights and permissions
About this article
Cite this article
Nakamura, T., Watanabe, M. Intestinal stem cell transplantation. J Gastroenterol 52, 151–157 (2017). https://doi.org/10.1007/s00535-016-1288-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1288-8