Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) presents as a spectrum ranging from simple steatosis to nonalcoholic steatohepatitis (NASH). The latter is progressive, and its pathogenesis remains poorly understood. Recently, bile acid (BA) metabolism has become a therapeutic focus in NASH patients. The aim of the present study was to explore changes in bile acid metabolism in NAFLD patients in the context of disease progression.
Methods
We prospectively enrolled patients with clinically suspected NAFLD. Patients taking ursodeoxycholic acid were excluded. The intrahepatic expression levels of genes associated with BA metabolism were determined by quantitative PCR and immunohistochemistry.
Results
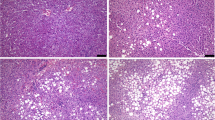
Seventy-eight patients (male:female = 49:29) histologically diagnosed with NAFLD were analyzed. The expression levels of farnesoid X receptor, liver receptor homolog 1, and small heterodimer partner, key proteins in BA synthesis, significantly decreased as the NAFLD activity score (NAS) increased in either males or females. The levels of cholesterol 7 alpha-hydroxylase, the rate-limiting enzyme of BA synthesis, were not changed. Notably, the expression levels of a main export transporter, bile salt export pump (BSEP), significantly decreased as the NAS and the each NAS component increased in both genders. The decreases of BSEP levels were also observed by immunohistochemistry, particularly in areas with pronounced fatty changes in cases with high NAS.
Conclusions
The expression levels of the BA export transporter BSEP were inversely correlated with NAS in NAFLD patients. Such down-regulation may cause excessive BA levels in hepatocytes, leading to cell injury. Our findings may afford new insights into the pathogenesis of NASH.






Similar content being viewed by others
Abbreviations
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- BA:
-
Bile acid
- FXR:
-
Farnesoid X receptor
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- UDCA:
-
Ursodeoxycholic acid
- OGTT:
-
Oral glucose tolerance test
- CRP:
-
C-reactive protein
- GGT:
-
Gamma-glutamyl transpeptidase
- PT-INR:
-
Prothrombin time-international normalized ratio
- FBG:
-
Fasting blood glucose
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- TG:
-
Triglyceride
- NAS:
-
NAFLD activity score
- BSEP:
-
Bile salt export pump
- MRP2:
-
Multidrug resistance-associated protein 2
- mRNA:
-
Messenger RNA
- SHP:
-
Small heterodimer partner
- LRH1:
-
Liver receptor homolog 1
- NTCP:
-
Na+/taurocholate cotransporter
- CYP7A1:
-
Cholesterol 7 alpha-hydroxylase
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- PFIC2:
-
Progressive familial intrahepatic cholestasis type 2
References
Kojima S, Watanabe N, Numata M, et al. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38(10):954–61.
Marrero JA, Fontana RJ, Su GL, et al. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36(6):1349–54.
Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56(6):1384–91.
Rubinstein E, Lavine JE, Schwimmer JB. Hepatic, cardiovascular, and endocrine outcomes of the histological subphenotypes of nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28(4):380–5.
Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65.
Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145(3):574–82 e1.
Trauner M, Claudel T, Fickert P, et al. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis. 2010;28(1):220–4.
Karpen SJ. Do therapeutic bile acids hit the sweet spot of glucose metabolism in NAFLD? Gastroenterology. 2013;145(3):508–10.
Mehal WZ. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10(11):637–44.
Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–6.
Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101.
Aranha MM, Cortez-Pinto H, Costa A, et al. Bile acid levels are increased in the liver of patients with steatohepatitis. Eur J Gastroenterol Hepatol. 2008;20(6):519–25.
Dasarathy S, Yang Y, McCullough AJ, et al. Elevated hepatic fatty acid oxidation, high plasma fibroblast growth factor 21, and fasting bile acids in nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2011;23(5):382–8.
Kalhan SC, Guo L, Edmison J, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;2011:404–13.
Ferslew BC, Johnston CK, Tsakalozou E, et al. Altered morphine glucuronide and bile acid disposition in patients with nonalcoholic steatohepatitis. Clin Pharmacol Ther. 2015;97(4):419–27.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23.
Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–9.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21.
Bechmann LP, Kocabayoglu P, Sowa JP, et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology. 2013;57(4):1394–406.
Kubitz R, Droge C, Kluge S, et al. Genetic variations of bile salt transporters. Drug Discov Today Technol. 2014;12:e55–67.
Strautnieks SS, Kagalwalla AF, Tanner MS, et al. Identification of a locus for progressive familial intrahepatic cholestasis PFIC2 on chromosome 2q24. Am J Hum Genet. 1997;61(3):630–3.
Kubitz R, Droge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol. 2015;48(2–3):273–84.
Lam P, Soroka CJ, Boyer JL. The bile salt export pump: clinical and experimental aspects of genetic and acquired cholestatic liver disease. Semin Liver Dis. 2010;30(2):125–33.
Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int. 2010;4(4):741–8.
Bjursell M, Wedin M, Admyre T, et al. Ageing Fxr deficient mice develop increased energy expenditure, improved glucose control and liver damage resembling NASH. PLoS One. 2013;8(5):e64721.
Kong B, Luyendyk JP, Tawfik O, et al. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328(1):116–22.
Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116(4):1102–9.
Xu JY, Li ZP, Zhang L, et al. Recent insights into farnesoid X receptor in non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20(37):13493–500.
Hayashi H, Sugiyama Y. 4-phenylbutyrate enhances the cell surface expression and the transport capacity of wild-type and mutated bile salt export pumps. Hepatology. 2007;45(6):1506–16.
Naoi S, Hayashi H, Inoue T, et al. Improved liver function and relieved pruritus after 4-phenylbutyrate therapy in a patient with progressive familial intrahepatic cholestasis type 2. J Pediatr. 2014;164(5):1219–27 e3.
Aguilar-Olivos NE, Carrillo-Cordova D, Oria-Hernandez J, et al. The nuclear receptor FXR, but not LXR, up-regulates bile acid transporter expression in non-alcoholic fatty liver disease. Ann Hepatol. 2015;14(4):487–93.
Acknowledgments
We thank Ms. Seiko Shinzawa (Department of Gastroenterology, Graduate School of Medicine, the University of Tokyo) for kind advice on research procedures. We thank Mr. Takeshi Shimamoto (Kameda Medical Center Makuhari) for detailed assistance with statistics. We also thank Mr. Kei Sakuma (Department of Pathology, Graduate School of Medicine, the University of Tokyo) for processing specimens for immunohistochemical analysis. This work was supported by Health Sciences Research Grants from the Ministry of Health, Labor, and Welfare of Japan (Research on Hepatitis) and AMED (the Japan Agency for Medical Research and Development). No additional external funding was received. The funders played no role in study design, data collection, or analysis, the decision to publish, or manuscript preparation.
Authors’ contributions
KO, TT, and KE contributed to the study concept and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. HF, AK, KM, and HY participated in critical revision of the manuscript to ensure that the intellectual content was of a high standard. JS and MF contributed to histological analysis. KK participated in study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript to ensure that the intellectual content was of a high standard, and study supervision. We confirm that all authors have reviewed and approved of the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
The associations between the changes of the expression levels of BA transporters and each component of the NAS in females. A, B, and C: Graded by steatosis. Significant associations were not evident. D, E, and F: Graded by lobular inflammation. BSEP and NTCP were significantly downregulated with progressions of lobular inflammation. G, H, and I: Graded by hepatocyte ballooning. Significant associations were not evident. (TIFF 71 kb)
Rights and permissions
About this article
Cite this article
Okushin, K., Tsutsumi, T., Enooku, K. et al. The intrahepatic expression levels of bile acid transporters are inversely correlated with the histological progression of nonalcoholic fatty liver disease. J Gastroenterol 51, 808–818 (2016). https://doi.org/10.1007/s00535-015-1148-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1148-y