Abstract
Background
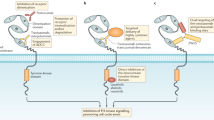
Pertuzumab is a humanized monoclonal antibody that binds to HER2 at an epitope that prevents HER2 from dimerizing with ligand-activated HER-family receptors. To assess the potential of pertuzumab as a new therapy, the expression status of HER family members was determined in biliary tract carcinoma (BTC), and the antitumor activity of pertuzumab was investigated by assessing the inhibition of BTC cell growth.
Methods
The expression status of HER family members in 113 archival specimens of BTC was analyzed by using immunohistochemistry and fluorescence in situ hybridization. Using ten BTC cell lines, heregulin-alpha (HRG-α) stimulated cell proliferation and its inhibition by pertuzumab was tested in vitro. The phosphorylated HER family proteins and their respective downstream molecules were analyzed. In vivo antitumor activity of pertuzumab was evaluated in a xenograft model.
Results
Protein overexpression of HER2 and/or HER3 was observed in 23–34 % of the specimens and gene amplification in 17–27 %. Seven of the ten cell lines showed HER2 and/or HER3 protein overexpression and gene amplification, and HRG-α stimulated cell proliferation was observed in four of the ten cell lines. In a BTC cell line co-overexpressing HER2 and HER3, pertuzumab potently inhibited the HRG-α stimulated cell proliferation in a dose-dependent manner, and completely blocked the phosphorylation of HER3. Suppression of downstream pathway molecules including p-AKT was also observed. Pertuzumab inhibited the in vivo growth of subcutaneous tumors, and increased the number of apoptotic cancer cells.
Conclusions
Pertuzumab exerts potent antitumor activity in BTC cells co-overexpressing HER2 and HER3. Pertuzumab provides a new therapeutic option against BTC.




Similar content being viewed by others
References
Dixon E, Vollmer CM Jr, Sahajpal A, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American center. Ann Surg. 2005;241:385–94.
Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–17.
Huang JL, Biehl TR, Lee FT, et al. Outcomes after resection of cholangiocellular carcinoma. Am J Surg. 2004;187:612–7.
Nakagohri T, Asano T, Kinoshita H, et al. Aggressive surgical resection for hilar-invasive and peripheral intrahepatic cholangiocarcinoma. World J Surg. 2003;27:289–93.
Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70:1493–7.
Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81.
Morizane C, Okusaka T, Mizusawa J, et al. Randomized phase II study of gemcitabine plus S-1 versus S-1 in advanced biliary tract cancer: a Japan Clinical Oncology Group trial (JCOG 0805). Cancer Sci. 2013;104:1211–6.
Sirica AE. Biliary proliferation and adaptation in furan-induced rat liver injury and carcinogenesis. Toxicol Pathol. 1996;24:90–9.
Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15.
Sirica AE, Lai GH, Endo K, et al. Cyclooxygenase-2 and ERBB-2 in cholangiocarcinoma: potential therapeutic targets. Semin Liver Dis. 2002;22:303–13.
Sirica AE, Lai GH, Zhang Z. Biliary cancer growth factor pathways, cyclo-oxygenase-2 and potential therapeutic strategies. J Gastroenterol Hepatol. 2001;16:363–72.
Sirica AE, Radaeva S, Caran N. NEU overexpression in the furan rat model of cholangiocarcinogenesis compared with biliary ductal cell hyperplasia. Am J Pathol. 1997;151:1685–94.
Kiguchi K, Carbajal S, Chan K, et al. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001;61:6971–6.
Sirica AE. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2008;14:7033–58.
Yarden Y, Sliwkowski MX. Untangling the ErbB signaling network. Nat Rev Mol Cell Biol. 2001;2:127–37.
Tanner KG, Kyte J. Dimerization of the extracellular domain of the receptor for epidermal growth factor containing the membrane-spanning segment in response to treatment with epidermal growth factor. J Biol Chem. 1999;274:35985–90.
Hsieh AC, Moasser MM. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer. 2007;97:453–7.
Park OK, Schaefer TS, Nathans D. In vitro activation of Stat3 by epidermal growth factor receptor kinase. Proc Natl Acad Sci USA. 1996;93:13704–8.
Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40.
Hynes NE, Lane HA. ErbB receptors and cancer: the complexity of targeted inhibitors. Nat Rev. 2005;5:341–54.
Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–28.
Agus DB, Gordon MS, Taylor C, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534–43.
Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–37.
Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–6.
Sakai K, Yokote H, Murakami-Murofushi K, et al. Pertuzumab, a novel HER dimerization inhibitor, inhibits the growth of human lung cancer cells mediated by the HER3 signaling pathway. Cancer Sci. 2007;98:1498–503.
Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft models. Cancer Res. 2009;69:9330–6.
Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19.
Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6th ed. Philadelphia: Lippincott-Raven; 2002. p. 139–50
Kawamoto T, Krishnamurthy S, Tarco E, et al. HER receptor family: novel candidate for targeted therapy for gallbladder and extrahepatic bile duct cancer. Gastrointest Cancer Res. 2007;1:221–7.
Iemura A, Maruiwa M, Yano H, et al. A new human cholangiocellular carcinoma cell line (KMC-1). J Hepatol. 1992;15:288–98.
Murakami T, Yano H, Maruiwa M, et al. Establishment and characterization of a human combined hepatocholangiocarcinoma cell line and its heterologous transplantation in nude mice. Hepatology. 1987;7:551–6.
Saito K, Minato H, Kono N, et al. Establishment of the human cholangiocellular carcinoma cell line (CCKS1). Kanzo. 1993;34:122–9 (in Japanese with English abstract).
Sripa B, Leungwattanawanit S, Nitta T, et al. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J Gastroenterol. 2005;11:3392–7.
Knuth A, Gabbert H, Dippold W, et al. Biliary adenocarcinoma. Characterization of three new human tumor cell lines. J Hepatol. 1985;1:579–96.
Koike N, Todoroki T, Kawamoto T, et al. The invasion potentials of human biliary tract carcinoma cell lines: correlation between invasiveness and morphologic characteristics. Int J Oncol. 1998;13:1269–74.
Sliwkowski MX, Schaefer G, Akita RW, et al. Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem. 1994;269:14661–5.
Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12.
Naidu R, Yadav M, Nair S, et al. Expression of c-erbB3 protein in primary breast carcinomas. Br J Cancer. 1998;78:1385–90.
Yukawa M, Fujimori T, Hirayama D, et al. Expression of oncogene products and growth factors in early gallbladder cancer, advanced gallbladder cancer, and chronic cholecystitis. Hum Pathol. 1993;24:37–40.
Nakazawa K, Dobashi Y, Suzuki S, et al. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol. 2005;206:356–65.
Altimari A, Fiorentino M, Gabusi E, et al. Investigation of ErbB1 and ErbB2 expression for therapeutic targeting in primary liver tumours. Dig Liver Dis. 2003;35:332–8.
Kalekou H, Miliaras D. Immunohistochemical study of microvessel density, CD44 (standard form), p53 protein and c-erbB2 in gallbladder carcinoma. J Gastroenterol Hepatol. 2004;19:812–8.
Muller-Tidow C, Diederichs S, Bulk E, et al. Identification of metastasis-associated receptor tyrosine kinases in non-small cell lung cancer. Cancer Res. 2005;65:1778–82.
Lebeau A, Deimling D, Kaltz C, et al. Her-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol. 2001;19:354–63.
Ellis CM, Dyson MJ, Stephenson TJ, et al. HER2 amplification status in breast cancer: a comparison between immunohistochemical staining and fluorescence in situ hybridization using manual and automated quantitative image analysis scoring techniques. J Clin Pathol. 2005;58:710–4.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82.
Seshadri R, Firgaira FA, Horsfall DJ, McCaul K, Setlur V, Kitchen P. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. J Clin Oncol. 1993;11:1936–42.
Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–28.
Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, Ochiai A, Ruschoff J, Henkei T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805.
Wiggers KJ, Ruys AT, Koerkamp BG, Beuers U, Ten Kate FJ, van Gulik TM. Differences in immunohistochemical biomarkers between intra- and extrahepatic cholangiocarcinoma: a systemic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:1582–94.
Graus-Porta D, Beerli RR, Daly JM, et al. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–55.
Hellyer NJ, Kim MS, Koland JG. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem. 2001;276:42153–61.
Jackson JG, St Clair P, Sliwkowski MX, et al. Blockade of epidermal growth factor- or heregulin-dependent ErbB2 activation with the anti-ErbB2 monoclonal antibody 2C4 has divergent downstream signaling and growth effects. Cancer Res. 2004;64:2601–9.
Yamashita-Kashima Y, Iijima S, Yorozu K, et al. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res. 2011;17:5060–70.
Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–44.
Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–63.
Acknowledgments
This study was supported by Grants-in-Aid (Nos. 15590618, 23390318, and 24390323) for scientific research from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawamoto, T., Ishige, K., Thomas, M. et al. Overexpression and gene amplification of EGFR, HER2, and HER3 in biliary tract carcinomas, and the possibility for therapy with the HER2-targeting antibody pertuzumab. J Gastroenterol 50, 467–479 (2015). https://doi.org/10.1007/s00535-014-0984-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-014-0984-5