Abstract
Background
In the respiratory mucosa, interleukin (IL)-33, has been shown to enhance T helper 2 (TH2)-type responses through the master regulatory gene GATA-3. IL-33 is upregulated in ulcerative colitis (UC), and the aim was to assess if IL-33 holds a similar key position in the shaping of the immune response in experimental colitis (piroxicam-accelerated colitis (PAC) in IL-10 −/− mice, dextran sodium sulfate (DSS) model) and UC.
Methods
Colonic IL-33 expression was determined in UC (8 active UC, 8 quiescent UC, and 7 controls) and experimental colitis. Mesenteric lymph node (MesLN) T cells were isolated from PAC IL-10 −/− mice and stimulated with IL-33.
Results
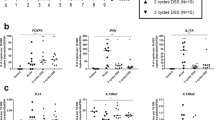
The colonic IL-33 expression was significantly upregulated all forms of colitis (P < 0.01) and correlated with disease severity score and inflammation (P < 0.001), and with GATA-3 expression levels (P < 0.01); no correlation with the TH1-specific T-bet expression was observed. MesLN T cells stimulated with IL-33 had increased GATA-3 expression, and showed an IL-33 dose-dependent increase in secreted TH2-type cytokines, whereas this effect was abolished by blocking IL-33 signaling. The non-TH2-type cytokine IL-17 was upregulated by IL-33 but in a T cell receptor dependent manner, as opposed to TH2-type cytokines, which required only IL-33 stimulation.
Conclusions
The study demonstrates that intestinal IL-33 is capable of inducing GATA-3 in mucosal T cells, and suggests that IL-33 is a key mediator of pathological TH2 and non-TH2-type responses in intestinal inflammation. Blocking IL-33 signaling could be a feasible option in the treatment of UC.






Similar content being viewed by others
References
Seidelin JB, Bjerrum JT, Coskun M, et al. IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett. 2010;128:80–5.
Pastorelli L, Garg RR, Hoang SB, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA. 2010;107:8017–22.
Sponheim J, Pollheimer J, Olsen T, et al. Inflammatory bowel disease-associated interleukin-33 is preferentially expressed in ulceration-associated myofibroblasts. Am J Pathol. 2010;177(6):2804–15.
Nile CJ, Barksby E, Jitprasertwong P, et al. Expression and regulation of interleukin-33 in human monocytes. Immunology. 2010;130:172–80.
Hudson CA, Christophi GP, Gruber RC, et al. Induction of IL-33 expression and activity in central nervous system glia. J Leukoc Biol. 2008;84:631–43.
Kobori A, Yagi Y, Imaeda H, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45(10):999–1007.
Seidelin JB, Rogler G, Nielsen OH. A role for interleukin-33 in T(H)2-polarized intestinal inflammation? Mucosal Immunol. 2011;4:496–502.
Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90.
Kurowska-Stolarska M, Kewin P, Murphy G, et al. IL-33 induces antigen-specific IL-5 + T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–90.
Komai-Koma M, Xu D, Li Y, et al. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–86.
Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4 + lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–70.
Fuss IJ, Heller F, Boirivant M, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–7.
Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58:1152–67.
Hamilton MJ, Snapper SB, Blumberg RS. Update on biologic pathways in inflammatory bowel disease and their therapeutic relevance. J Gastroenterol. 2012;47:1–8.
Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89.
Vahedi G, Kanno Y, Sartorelli V, O’Shea JJ. Transcription factors and CD4 T cells seeking identity: masters, minions, setters and spikers. Immunology. 2013;139:294–8.
Ben-Sasson SZ, Le GG, Conrad DH, et al. IL-4 production by T cells from naive donors. IL-2 is required for IL-4 production. J Immunol. 1990;145:1127–36.
Zhu J, Yamane H, Cote-Sierra J, et al. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16:3–10.
Le GG, Ben-Sasson SZ, Seder R, et al. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–9.
Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17 + T helper cells. Cell. 2006;126:1121–33.
Szabo SJ, Kim ST, Costa GL, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69.
Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90.
Hong J, Bae S, Jhun H, et al. Identification of constitutively active interleukin 33 (IL-33) splice variant. J Biol Chem. 2011;286:20078–86.
Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–6.
Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009;284:19420–6.
Luthi AU, Cullen SP, McNeela EA, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98.
Lopetuso LR, Chowdhry S, Pizarro TT. Opposing functions of classic and novel IL-1 family members in gut health and disease. Front Immunol. 2013;4:181.
Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49.
Okayasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702.
Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–67.
Wilson MS, Ramalingam TR, Rivollier A, et al. Colitis and intestinal inflammation in IL10−/− mice results from IL-13Ralpha2-mediated attenuation of IL-13 activity. Gastroenterology. 2011;140:254–64.
Holgersen K, Kvist PH, Markholst H, et al. Characterisation of enterocolitis in the piroxicam-accelerated interleukin-10 knock out mouse—a model mimicking inflammatory bowel disease. J Crohns Colitis. 2013;8(2):147–60.
Holgersen K, Kvist PH, Hansen AK, Holm TL. Predictive validity and immune cell involvement in the pathogenesis of piroxicam-accelerated colitis in interleukin-10 knockout mice. Int Immunopharmacol. 2014;21:137–47.
Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–9.
Langholz E, Munkholm P, Davidsen M, et al. Changes in extent of ulcerative colitis: a study on the course and prognostic factors. Scand J Gastroenterol. 1996;31:260–6.
Langholz E. Ulcerative colitis. An epidemiological study based on a regional inception cohort, with special reference to disease course and prognosis. Dan Med Bull. 1999;46:400–15.
Kjellev S, Thim L, Pyke C, Poulsen SS. Cellular localization, binding sites, and pharmacologic effects of TFF3 in experimental colitis in mice. Dig Dis Sci. 2007;52:1050–9.
Murthy SN, Cooper HS, Shim H, et al. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–34.
Bleich A, Mahler M, Most C, et al. Refined histopathologic scoring system improves power to detect colitis QTL in mice. Mamm Genome. 2004;15:865–71.
Beltrán CJ, Núñez LE, Díaz-Jiménez D, et al. Characterization of the novel ST2/IL-33 system in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1097–107.
Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol. 1995;101:428–35.
Specht S, Arriens S, Hoerauf A. Induction of chronic colitis in IL-10 deficient mice requires IL-4. Microbes Infect. 2006;8:694–703.
Pushparaj PN, Tay HK, H’ng SC, et al. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci USA. 2009;106:9773–8.
Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35.
Chackerian AA, Oldham ER, Murphy EE, et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–5.
Greenfeder SA, Nunes P, Kwee L, et al. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J Biol Chem. 1995;270:13757–65.
Funakoshi-Tago M, Tago K, Hayakawa M, et al. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell Signal. 2008;20:1679–86.
Guo L, Wei G, Zhu J, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci USA. 2009;106:13463–8.
Halim TY, Krauss RH, Sun AC, Takei F. VLung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–63.
Oboki K, Ohno T, Kajiwara N, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA. 2010;107:18581–6.
Sartor RB. Innate immunity in the pathogenesis and therapy of IBD. J Gastroenterol. 2003;38(Suppl 15):43–7.
Wang ZY, Kusam S, Munugalavadla V, et al. Regulation of Th2 cytokine expression in NKT cells: unconventional use of Stat6, GATA-3, and NFAT2. J Immunol. 2006;176:880–8.
Sehra S, Patel D, Kusam S, et al. A role for caspases in controlling IL-4 expression in T cells. J Immunol. 2005;174:3440–6.
Duan L, Chen J, Zhang H, et al. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3(+) regulatory T-cell responses in mice. Mol Med. 2012;18:753–61.
Pushparaj PN, Li D, Komai-Koma M, et al. Interleukin-33 exacerbates acute colitis via interleukin-4 in mice. Immunology. 2013;140(1):70–7.
Grobeta P, Doser K, Falk W, et al. IL-33 attenuates development and perpetuation of chronic intestinal inflammation. Inflamm Bowel Dis. 2012;18:1900–9.
Sedhom MA, Pichery M, Murdoch JR, et al. Neutralisation of the interleukin-33/ST2 pathway ameliorates experimental colitis through enhancement of mucosal healing in mice. Gut. 2012;62(12):1714–23.
Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–40.
Acknowledgments
We thank Anders Hansen, Camilla Frost Sørensen, Hanne Fuglsang, Jeanette Juul, Lotte Friis, and Vibeke Voxen Hansen for excellent technical assistance. This study was supported by grants from Fonden til Lægevidenskabens Fremme (the A. P. Møller Foundation), Novo Nordisk A/S, the Family Erichsen Memorial Foundation, the Lundbeck Foundation, the Axel Muusfeldts Foundation, and the Foundation of Aase and Ejnar Danielsen. JS holds a grant from the Danish Council for Independent Research.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. B. Seidelin and M. Coskun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Seidelin, J.B., Coskun, M., Kvist, P.H. et al. IL-33 promotes GATA-3 polarization of gut-derived T cells in experimental and ulcerative colitis. J Gastroenterol 50, 180–190 (2015). https://doi.org/10.1007/s00535-014-0982-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-014-0982-7