Abstract
Background
This study explores pretreatment predictive factors for ultimate virological responses to pegylated interferon-α (1.5 μg/kg/week) and ribavirin (600–1000 mg/day) (PEG-IFN/RBV) combination therapy for patients infected with hepatitis C virus (HCV)-1b and a high viral load.
Methods
A total of 75 patients underwent PEG-IFN/RBV combination therapy for 48 weeks. HCV amino acid (aa) substitutions in non-structural protein 5a, including those in the IFN/RBV resistance-determining region (IRRDR) and the IFN sensitivity-determining region and the core regions, as well as the genetic variation (rs8099917) near the interleukin 28B (IL28B) gene (genotype TT) were analyzed.
Results
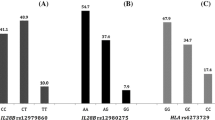
Of the 75 patients, 49 % (37/75) achieved a sustained virological response (SVR), 27 % (20/75) showed relapse, and 24 % (18/75) showed null virological response (NVR). Multivariate logistic regression analysis identified IRRDR with 6 or more mutations (IRRDR ≥6) [odds ratio (OR) 11.906, p < 0.0001] and age <60 years (OR 0.228, p = 0.015) as significant determiners of SVR and IL28B minor (OR 14.618, p = 0.0019) and platelets <15 × 104/mm3 (OR 0.113, p = 0.0096) as significant determiners of NVR. A combination of IRRDR ≥6 and age <60 years improved SVR predictability (93.3 %), and that of IRRDR ≤5 and age ≥60 years improved non-SVR predictability (84.0 %). Similarly, a combination of IL28B minor and platelets <15 × 104/mm3 improved NVR predictability (85.7 %), and that of IL28B major and platelets ≥15 × 104/mm3 improved non-NVR (response) (97.1 %) predictability.
Conclusion
IRRDR ≥6 and age <60 years were significantly associated with SVR. IL28B minor and platelets <15 × 104/mm3 were significantly associated with NVR. Certain combinations of these factors improved SVR and NVR predictability and could, therefore, be used to design therapeutic strategies.
Similar content being viewed by others
References
Bialek SR, Terrault NA. The changing epidemiology and natural history of hepatitis C virus infection. Clin Liver Dis. 2006;10:697–715.
Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–6.
Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82.
Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46:37–47.
Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–52.
Kurosaki M, Sakamoto N, Iwasaki M, Sakamoto M, Suzuki Y, Hiramatsu N, et al. Pretreatment prediction of response to peginterferon plus ribavirin therapy in genotype 1 chronic hepatitis C using data mining analysis. J Gastroenterol. 2011;46:401–9.
Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-α and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–9.
Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401.
Suppiah V, Moldovan M, Ahlenstiel G, Ber T, Weltman M, Abate ML, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1105–9.
Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure—a genome-wide association study. Gastroenterology. 2010;138:1338–45.
El-Shamy A, Sasayama M, Nagano-Fujii M, Sasase N, Imoto S, Kim SR, et al. Prediction of efficient virological response to pegylated interferon/ribavirin combination therapy by NS5A sequences of hepatitis C virus and anti-NS5A antibodies in pre-treatment sera. Microbiol Immunol. 2007;51:471–82.
El-Shamy A, Nagano-Fujii M, Sasase N, Imoto S, Kim SR, Hotta H. Sequence variation in hepatitis C virus nonstructural protein 5A predicts clinical outcome of pegylated interferon/ribavirin combination therapy. Hepatology. 2008;48:38–47.
El-Shamy A, Kim SR, Ide YH, Sasase N, Imoto S, Deng L, et al. Polymorphisms of hepatitis C virus non-structural protein 5A and core protein and clinical outcome of pegylated-interferon/ribavirin combination therapy. Intervirology. 2012;55:1–11.
Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, et al. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N Engl J Med. 1996;11(334):77–81.
Akuta N, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, et al. Predictive factors of early and sustained responses to peginterferon plus ribavirin combination therapy in Japanese patients infected with hepatitis C virus genotype 1b: amino acid substitutions in the core region and low-density lipoprotein cholesterol levels. J Hepatol. 2007;6:403–10.
Akuta N, Suzuki F, Sezaki H, Suzuki Y, Hosaka T, Someya T, et al. Association of amino acid substitution pattern in core protein of hepatitis C virus genotype 1b high viral load and non-virological response to interferon–ribavirin combination therapy. Intervirology. 2005;48:372–80.
Okamoto H, Sugiyama Y, Okada S, Kurai K, Akahane Y, Sugai Y, et al. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992;73:673–9.
Ogata S, Nagano-Fujii M, Ku Y, Yoon S, Hotta H. Comparative sequence analysis of the core protein and its frameshift product, the F protein, of hepatitis C virus subtype 1b strains obtained from patients with and without hepatocellular carcinoma. J Clin Microbiol. 2002;40:3625–30.
Fukuhara T, Taketomi A, Okano S, Ikegami T, Soejima Y, Shirabe K, et al. Mutations in hepatitis C virus genotype 1b and the sensitivity of interferon–ribavirin therapy after liver transplantation. J Hepatol. 2010;52:672–80.
Hayes CN, Kobayashi M, Akuta N, Suzuki F, Kumada H, Abe H, et al. HCV substitutions and IL28B polymorphisms on outcome of peg-interferon plus ribavirin combination therapy. Gut. 2011;60:261–7.
Kurosaki M, Tanaka Y, Nishida N, Sakamoto N, Enomoto N, Honda M, et al. Pre-treatment prediction of response to pegylated-interferon plus ribavirin for chronic hepatitis C using genetic polymorphism in IL28B and viral factors. J Hepatol. 2011;54:439–48.
Macdonald A, Harris M. Hepatitis C virus NS5A: tales of a promiscuous protein. J Gen Virol. 2004;85:2485–502.
El-Shamy A, Shoji I, Kim SR, Ide Y, Imoto S, Deng L, et al. Sequence heterogeneity in NS5A of hepatitis C virus genotypes 2a and 2b and clinical outcome of pegylated-interferon/ribavirin therapy. PLoS One. 2012;7(2):e30513.
Tsai YH, Kuang WF, Lu TY, Kao JH, Lai MY, Liu CJ, et al. The non-structural 5A protein of hepatitis C virus exhibits genotypic differences in interferon antagonism. J Hepatol. 2008;49:899–907.
Hughes M, Gretton S, Shelton H, Brown DD, McCormick CJ, Angus AG, et al. A conserved proline between domains II and III of hepatitis C virus NS5A influences both RNA replication and virus assembly. J Virol. 2009;83:10788–96.
Tellinghuisen TL, Foss KL, Treadaway J. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 2008;4:e1000032.
Vlotides G, Sorensen AS, Kopp F, Zitzmann K, Cengic N, Brand S, et al. SOCS-1 and SOCS-3 inhibit IFN-α-induced expression of the antiviral proteins 2,5-OAS and MxA. Biochem Biophys Res Commun. 2004;320:1007–14.
Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77.
Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–8.
Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–98.
Berg T, von Wagner M, Nasser S, Sarrazin C, Heintges T, Gerlach T, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006;130:1086–97.
McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–38.
Hézode C, Forestier N, Dusheiko G, Ferenci P, Plo S, Goeser T, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839–50.
Acknowledgments
This work was supported in part by Grants-in-Aid for Research on Hepatitis from the Ministry of Health, Labor and Welfare, Japan, and a SATREPS Grant from JST and JICA. The study was also carried out as part of the J-GRID Program, Ministry of Education, Culture, Sports, Science and Technology, Japan, and the G-COE Program at Kobe University Graduate School of Medicine. We are indebted to Ms. Yoshiko Kawamura of Kobe Asahi Hospital for assistance in the preparation of the manuscript.
Conflict of interest
None of the authors has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S.R., El-Shamy, A., Imoto, S. et al. Prediction of response to pegylated interferon/ribavirin combination therapy for chronic hepatitis C genotype 1b and high viral load. J Gastroenterol 47, 1143–1151 (2012). https://doi.org/10.1007/s00535-012-0578-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-012-0578-z