Abstract
Background
Intrahepatic cholangiocarcinoma (ICC) is rising in clinical importance due to the increasing incidence worldwide, poor prognosis, and suboptimal response to therapies. New effective therapeutic approaches are needed for improvement of treatment outcome. A recent study showed that sorafenib, a multikinase inhibitor that acts predominantly through inhibition of Raf kinase and vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptors, exhibited potent antitumor activity in a preclinical model of cholangiocarcinoma cells.
Method
We tested the in vitro and in vivo antitumor activity of sorafenib against human ICC cell lines.
Results
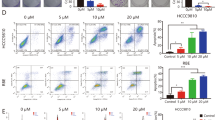
Treatment of ICC cells with sorafenib resulted in inhibition of proliferation and induction of apoptosis in the cell lines. In the cells treated with sorafenib, phosphorylation of mitogen-activated protein kinase kinase (MEK) and mitogen-activated protein kinase (MAPK) and also interleukin-6-induced phosphorylation of signal transducer and activator of transcription 3 (STAT3) were inhibited in a dose-dependent manner. Down-regulation of the anti-apoptotic protein myeloid cell leukemia-1 (Mcl-1) paralleled the reduced phosphorylation of STAT3. However, sorafenib induced no significant change in the cell cycle distribution and the expression levels of cyclin D1 and p27Kip1 in the cells. For the in vivo antitumor activity, oral administration of sorafenib significantly inhibited the growth of subcutaneous tumors established in immunodeficient mice at doses of 10, 30, and 100 mg/kg. Moreover, administration of sorafenib (30 mg/kg) to animals with peritoneally disseminated ICC resulted in significantly prolonged survival compared with that of untreated animals (76 vs. 43 days in treated and vehicle-treated mice, respectively).
Conclusion
These results indicate that sorafenib is a potent agent that may provide a new therapeutic option for human ICC.






Similar content being viewed by others
Abbreviations
- Ab:
-
Antibody
- AKT/PKB:
-
Protein kinase B
- CC:
-
Cholangiocarcinoma
- IH:
-
Intrahepatic
- IL-6:
-
Interleukin-6
- JAK:
-
Janus kinase
- MAPK:
-
Mitogen-activated protein kinase
- Mcl-1:
-
Myeloid cell leukemia-1
- MEK:
-
Mitogen-activated protein kinase kinase
- STAT:
-
Signal transducer and activator of transcription
References
Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–21.
Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–14.
Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–67.
Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–17 (discussion 517–9).
Huang JL, Biehl TR, Lee FT, Zimmer PW, Ryan JA Jr. Outcomes after resection of cholangiocellular carcinoma. Am J Surg. 2004;187:612–7.
Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res. 2007;5:203–20.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109.
Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–40.
Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–72.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Bengala C, Bertolini F, Malavasi N, Boni C, Aitini E, Dealis C, et al. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer. 2010;102:68–72.
Huether A, Hopfner M, Baradari V, Schuppan D, Scherubl H. Sorafenib alone or as combination therapy for growth control of cholangiocarcinoma. Biochem Pharmacol. 2007;73:1308–17.
Iemura A, Maruiwa M, Yano H, Kojiro M. A new human cholangiocellular carcinoma cell line (KMC-1). J Hepatol. 1992;15:288–98.
Murakami T, Yano H, Maruiwa M, Sugihara S, Kojiro M. Establishment and characterization of a human combined hepatocholangiocarcinoma cell line and its heterologous transplantation in nude mice. Hepatology. 1987;7:551–6.
Saito K, Minato H, Kono N, Nakanuma Y, Ishida F, Kosugi M. Establishment of the human cholangiocellular carcinoma cell line (CCKS1). Kanzo. 1993;34:122–9 (in Japanese).
Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, et al. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J Gastroenterol. 2005;11:3392–7.
Tepsiri N, Chaturat L, Sripa B, Namwat W, Wongkham S, Bhudhisawasdi V, et al. Drug sensitivity and drug resistance profiles of human intrahepatic cholangiocarcinoma cell lines. World J Gastroenterol. 2005;11:2748–53.
Nabekura T, Otsu M, Nagasawa T, Nakauchi H, Onodera M. Potent vaccine therapy with dendritic cells genetically modified by the gene-silencing-resistant retroviral vector GCDNsap. Mol Ther. 2006;13:301–9.
Suzuki A, Obi K, Urabe T, Hayakawa H, Yamada M, Kaneko S, et al. Feasibility of ex vivo gene therapy for neurological disorders using the new retroviral vector GCDNsap packaged in the vesicular stomatitis virus G protein. J Neurochem. 2002;82:953–60.
Ishige K, Shoda J, Kawamoto T, Matsuda S, Ueda T, Hyodo I, et al. Potent in vitro and in vivo antitumor activity of interleukin-4-conjugated Pseudomonas exotoxin against human biliary tract carcinoma. Int J Cancer. 2008;123:2915–22.
Hirai H, Roussel MF, Kato JY, Ashmun RA, Sherr CJ. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995;15:2672–81.
Kato JY, Matsuoka M, Polyak K, Massague J, Sherr CJ. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–96.
Blechacz BR, Smoot RL, Bronk SF, Werneburg NW, Sirica AE, Gores GJ. Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. Hepatology. 2009;50:1861–70.
Isomoto H, Kobayashi S, Werneburg NW, Bronk SF, Guicciardi ME, Frank DA, et al. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology. 2005;42:1329–38.
Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8.
Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105.
Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–56.
Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, et al. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–69.
Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20.
Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102.
Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13.
Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054–65.
Harder J, Riecken B, Kummer O, Lohrmann C, Otto F, Usadel H, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer. 2006;95:848–52.
Kim ST, Park JO, Lee J, Lee KT, Lee JK, Choi SH, et al. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006;106:1339–46.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (20390339, 22390379, 22591530).
Conflict of interest
All authors declare that they have no conflict of interests or financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugiyama, H., Onuki, K., Ishige, K. et al. Potent in vitro and in vivo antitumor activity of sorafenib against human intrahepatic cholangiocarcinoma cells. J Gastroenterol 46, 779–789 (2011). https://doi.org/10.1007/s00535-011-0380-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-011-0380-3