Abstract
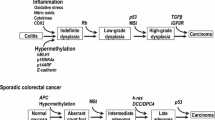
Chronic inflammatory bowel disease (IBD) is an important etiologic factor in the development of colorectal cancer. However, the mechanism underlying the development of colorectal cancers through chronic inflammation is not known. Activation-induced cytidine deaminase (AID) was originally identified as an inducer of somatic hypermutation in the immunoglobulin gene. We recently found that the mutagenic activity of AID expression links inflammation to the development of cancer. Aberrant AID expression is triggered by hepatitis C virus infection in human hepatocytes or Helicobacter pylori infection in human gastric epithelial cells, and leads to the generation of somatic mutations in various tumor-related genes. Here, we review our findings relating to how AID contributes to the development of colitis-associated colorectal cancers (CACs). Immunohistochemistry revealed the enhanced expression of endogenous AID protein in not only in the inflamed colonic mucosa of ulcerative colitis patients but also CAC tumor lesions. Pro-inflammatory cytokine TNF-α induced strong aberrant expression of AID via IκB kinase-dependent NF-κB-signaling pathways in human colonic epithelial cells. Furthermore, AID expression was also elicited in response to the T helper cell-2-driven cytokines IL-4 and IL-13, which are activated in human IBD. Aberrant activation of AID in colonic cells preferentially evoked genetic mutations in the TP53 gene, whereas there were no nucleotide alterations of the APC gene. These findings suggested that pro-inflammatory cytokine-mediated aberrant expression of AID in colonic epithelial cells plays a role as a genotoxic factor that enhances genetic instability during chronic colonic inflammation, leading to CAC development.



Similar content being viewed by others
Abbreviations
- AID:
-
Activation-induced cytidine deaminase
- TNF:
-
Tumor necrosis factor
- NF-κB:
-
Nuclear factor κB
- IKK:
-
IκB kinase
- IL:
-
Interleukin
References
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29.
Mellemkjaer L, Olsen JH, Frisch M, Johansen C, Gridley G, McLaughlin JK. Cancer in patients with ulcerative colitis. Int J Cancer. 1995;60:330–3.
Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35.
Lennard-Jones JE, Morson BC, Ritchie JK, Williams CB. Cancer surveillance in ulcerative colitis. Experience over 15 years. Lancet. 1983;2:149–52.
Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724–9.
Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–2.
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67.
Yin J, Harpaz N, Tong Y, Huang Y, Laurin J, Greenwald BD, et al. p53 point mutations in dysplastic and cancerous ulcerative colitis lesions. Gastroenterology. 1993;104:1633–9.
Kern SE, Redston M, Seymour AB, Caldas C, Powell SM, Kornacki S, et al. Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology. 1994;107:420–8.
Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–6.
Kou T, Marusawa H, Kinoshita K, Endo Y, Okazaki IM, Ueda Y, et al. Expression of activation-induced cytidine deaminase in human hepatocytes during hepatocarcinogenesis. Int J Cancer. 2007;120:469–76.
Endo Y, Marusawa H, Kinoshita K, Morisawa T, Sakurai T, Okazaki IM, et al. Expression of activation-induced cytidine deaminase in human hepatocytes via NF-kappaB signaling. Oncogene. 2007;26:5587–95.
Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63.
Kinoshita K, Nonaka T. The dark side of activation-induced cytidine deaminase: relationship with leukemia and beyond. Int J Hematol. 2006;83:201–7.
Okazaki IM, Hiai H, Kakazu N, Yamada S, Muramatsu M, Kinoshita K, et al. Constitutive expression of AID leads to tumorigenesis. J Exp Med. 2003;197:1173–81.
Morisawa T, Marusawa H, Ueda Y, Iwai A, Okazaki IM, Honjo T, et al. Organ-specific profiles of genetic changes in cancers caused by activation-induced cytidine deaminase expression. Int J Cancer. 2008;123:2735–40.
Chiba T, Marusawa H, Seno H, Watanabe N. Mechanism for gastric cancer development by Helicobacter pylori infection. J Gastroenterol Hepatol. 2008;23:1175–81.
Komori J, Marusawa H, Machimoto T, Endo Y, Kinoshita K, Kou T, et al. Activation-induced cytidine deaminase links bile duct inflammation to human cholangiocarcinoma. Hepatology. 2008;47:888–96.
Endo Y, Marusawa H, Kou T, Nakase H, Fujii S, Fujimori T, et al. Activation-induced cytidine deaminase links between inflammation and the development of colitis-associated colorectal cancers. Gastroenterology 2008;135:889–98, 898 e1–3.
Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17:173–88.
Dedeoglu F, Horwitz B, Chaudhuri J, Alt FW, Geha RS. Induction of activation-induced cytidine deaminase gene expression by IL-4 and CD40 ligation is dependent on STAT6 and NFkappaB. Int Immunol. 2004;16:395–404.
Conflict of interest
No conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Endo, Y., Marusawa, H. & Chiba, T. Involvement of activation-induced cytidine deaminase in the development of colitis-associated colorectal cancers. J Gastroenterol 46 (Suppl 1), 6–10 (2011). https://doi.org/10.1007/s00535-010-0326-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-010-0326-1