Abstract
Background
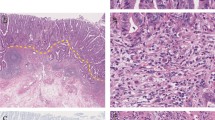
We have previously demonstrated a relationship between the depth of submucosal invasion (SM depth) and the frequency of lymph node metastasis in resected submucosal invasive colorectal cancers (SICRCs). Here, we assessed the desmoplastic reaction (DR) in pretreatment biopsy specimens of SICRC to predict the SM depth.
Methods
A total of 359 patients with SICRCs, who had undergone surgical or endoscopic mucosal resection, were enrolled. The SM depth of the SICRC lesions was evaluated according to the procedure established by the Japanese Society for Cancer of the Colon and Rectum, and the patients’ corresponding pretreatment biopsy specimens were examined histologically to evaluate the prevalence of DR.
Results
For pedunculated SICRCs, the prevalence of DR in pretreatment biopsy specimens was significantly higher in moderately differentiated than in well-differentiated adenocarcinomas, but was not significantly related to SM depth. For nonpedunculated SICRCs, the prevalence of DR in pretreatment biopsy specimens was significantly related to histological type, tumor size, and SM depth. When non-pedunculated SICRCs were further divided using a specific cutoff value of 1000 μm for SM depth, the DR positivity rate in pretreatment biopsy specimens was significantly higher in SICRCs with an SM depth of ≧1000 μm (termed “SM massive CRCs”) than in cases where the SM depth was <1000 μm (termed “SM slight CRCs”).
Conclusions
Detection of DR in pretreatment biopsy specimens is useful for the prediction of SM depth in nonpedunculated SICRCs, and may be useful for the selection of such cases that would be treatable by endoscopic mucosal resection and endoscopic submucosal dissection (EMR/ESD).

Similar content being viewed by others
References
Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534–43.
Coverlizza S, Risio M, Ferrari A, Fenoglio-Preiser CM, Rossini FP. Colorectal adenomas containing invasive carcinoma: pathologic assessment of lymph node metastatic potential. Cancer. 1989;64:1937–47.
Nivatvongs S, Rojanasakul A, Reiman HM, Dozois RR, Wolff BG, Pemberton JH, et al. The risk of lymph node metastasis in colorectal polyps with invasive adenocarcinoma. Dis Colon Rectum. 1991;34:323–8.
Netzer P, Forster C, Biral R, Ruchti C, Neuweiler J, Stauffer E, et al. Risk factor assessment of endoscopically removed malignant polyps. Gut. 1998;43:669–74.
Wang HS, Liang WY, Lin TC, Chen WS, Jiang JK, Yang SH, et al. Curative resection of T1 colorectal carcinoma: risk of lymph node metastasis and long-term prognosis. Dis Colon Rectum. 2005;48:1182–92.
Hassan C, Zullo A, Risio M, Rossini FP, Morini S. Histologic risk factors and clinical outcome in colorectal malignant polyp: a pooled-data analysis. Dis Colon Rectum. 2005;48:1588–96.
Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–12.
Kawamura YJ, Sakuragi M, Togashi K, Okuda M, Nagai H, Konishi F. Distribution of lymph node metastasis in T1 sigmoid colon carcinoma. Scand J Gastroenterol. 2005;40:858–61.
Schurch W, Seemayer TA, Gabbiani G. Myofibroblasts. In: Sternberg SS, editor. Histology for pathologists. 2nd ed. Philadelphia: Lippincott-Raven Publishers; 1997. p. 129–65.
Martin M, Pujuguet P, Martin F. Role of stromal myofibroblast. Pathol Res Pract. 1996;192:708–11.
Halvorsen TB, Seim EVA. Association between invasiveness, inflammatory reaction, desmoplasia and survival in colorectal cancer. J Clin Pathol. 1989;42:162–6.
Sis B, Sarioglu S, Sokmen S, Sakar M, Kupelioglu A, Fuzun M. Desmoplasia measured by computer assisted image analysis: an independent prognostic marker in colorectal carcinoma. J Clin Pathol. 2005;58:32–8.
Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082–90.
Nakada I, Tasaki T, Ubukata H, Goto Y, Watanabe Y, Sato S, et al. Desmoplastic response in biopsy specimens of early colorectal carcinoma is predictive of deep submucosal invasion. Dis Colon Rectum. 1998;41:896–900.
Japanese Society for Cancer of the Colon and Rectum. General rules for clinical and pathological studies on cancer of the colon, rectum and anus. 7th ed., revised version. Tokyo: Kanehara; 2009. p. 41–2.
Jass JR, Sobin LH. Histological typing of intestinal tumors. In: World Health Organization, editor. International histological classification of tumors. 2nd ed. Berlin: Springer; 1989. p. 29–40.
Ohtani H, Sasano N. Stromal cell changes in human colorectal adenomas and carcinomas: an ultrastructural study of fibroblasts, myofibroblasts, and smooth muscle cells. Virchows Arch. 1983;401:209–22.
Yao T, Tsuneyoshi M. Significance of pericryptal fibroblasts in colorectal epithelial tumors: a special reference to the histologic features and growth patterns. Hum Pathol. 1993;24:525–33.
Jacoby RF, Schlack S, Cole CE, Skarbek M, Harris C, Meisner LF. A juvenile polyposis tumor suppressor locus at 10q22 is deleted from nonepithelial cells in the lamina propria. Gastroenterology. 1997;112:1398–403.
Kinzler KW, Vogelstein B. Landscaping the cancer terrain. Science. 1998;280:1036–7.
Brosens LAA, Montgomery EA, Bhagavan BS, Offerhaus GJA, Giardiello FM. Mucosal prolapse syndrome presenting as rectal polyposis. J Clin Pathol. 2009;62:1034–6.
Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277:C183–201.
Tiitta O, Shipponen P, Gould V, Virtanen I. Tenascin expression in inflammatory, dysplastic and neoplastic lesions of the human stomach. Virchows Arch. 1994;425:369–74.
Yoshiro M, Chung YS, Kubo T, Hato F, Sowa M. Differential responses of scirrhous and well-differentiated gastric cancer cells to orthotopic fibroblasts. Br J Cancer. 1996;74:1096–103.
Hewitt RE, Desmond G, Powe G, Carter I, Turner DR. Desmoplasia and its relevance to colorectal tumour invasion. Int J Cancer. 1993;53:62–9.
Conti JA, Kendall TJ, Bateman A, Armstrong TA, Papa-Adams A, Xu Q, et al. The desmoplastic reaction surrounding hepatic colorectal adenocarcinoma metastases aids tumor growth and survival via αv integrin ligation. Clin Cancer Res. 2008;14:6405–13.
Martin M, Pujuguet P, Martin F. Role of stromal myofibroblasts infiltrating colon cancer in tumor invasion. Pathol Res Pract. 1996;192:712–7.
Pujuguet P, Hammann A, Moutet M, Samuel JL, Martin F, Martin M. Expression of fibronectin ED-A+ abd ED-B+ isoforms by human and experimental colorectal cancer. Contribution of cancer cells and tumor-associated myofibroblasts. Am J Pathol. 1996;148:579–92.
Acknowledgments
We thank Drs. Shigehiko Fujii (Kyoto-Katsura Hospital), Takahiro Fujii (TF Clinic), Yasushi Sano (Gastrointestinal Center, Sano Hospital), Shinji Tanaka (Department of Endoscopy, Hiroshima University), and Tetsuichiro Muto (Cancer Institute Hospital) for their valuable advice. This study was supported by the Japanese Society for Cancer of the Colon and Rectum.
Conflict of interest
No conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirose, M., Fukui, H., Igarashi, Y. et al. Detection of desmoplastic reaction in biopsy specimens is useful for predicting the depth of invasion of early colorectal cancer: a Japanese collaborative study. J Gastroenterol 45, 1212–1218 (2010). https://doi.org/10.1007/s00535-010-0288-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-010-0288-3