Abstract
Background
The aim of the study was to estimate the effect of viral factors (HBV genotype, viral load and kinetics) to treatment response in chronic hepatitis B (CHB) and first-line therapy with adefovir dipivoxil (ADV).
Methods
Sixty-six patients (60% males, 65% HBeAg negative) were treated with 10 mg ADV QD. Quantitative HBV DNA and ALT levels were determined at weeks 4, 12, 24, 48, 72 and 96. Nonresponse or viral resistance to ADV was assessed in patients with either persistent elevated HBV DNA levels (week 24) or with an increase in HBV DNA of at least 1 log after initial decline.
Results
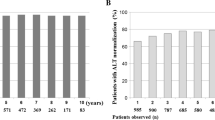
Most patients were infected with genotype D (66.7%; genotype A: 27.3%; genotype E: 6%); 86.4% achieved a virological (VR) and 54.5% a biochemical response (BR) in week 48, more often in patients with genotype A (P < 0.01). In week 96, BR increased to 60.5%, whereas a negative HBV DNA was observed in 83.3%. In 3% an ADV-induced viral resistance was detected. As an important predictive parameter for VR, a rapid decline of viral load at week 12 was observed. Of the patients with a negative PCR or drop of viral load of at least 3 log, 96% were still HBV DNA negative at the end of week 96; 77% of patients with a partial response achieved a VR. In contrast, no patient with nonresponse (week 12) reached a negative PCR at week 96 (P < 0.0001).
Conclusions
These results underline the importance of early viral kinetics to assess treatment response in CHB. In ADV nonresponders (week 12), an advanced antiviral therapy or switch to another nucleoside analogue should be considered.




Similar content being viewed by others
References
Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34:1225–41.
Maddrey WC. Hepatitis B: an important public health issue. J Med Virol. 2000;61:362–6.
Chang CN, Skalski V, Hua Zhou J, Cheng YC. Biochemical pharmacology of (+)-and (−)-2′,3′-dideoxy-3′-thiacytidine as anti-hepatitis B virus agents. J Biol Chem. 1992;267:22414–20.
Tassopoulos NC, Volpes R, Pastore G, Heathcote J, Buti M, Goldin RD, et al. Efficacy of lamivudine in patients with hepatitis B e antigen-negative/hepatitis B virus DNA positive (precore mutant) chronic hepatitis B. Hepatology. 1999;29:889–96.
Leung N. Treatment of chronic hepatitis B: case selection and duration of therapy. J Gastroenterol Hepatol. 2002;17:409–14.
Lau DT, Khokhar F, Doo E, Ghany MG, Herion D, Park Y, et al. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000;32:828–34.
Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Gastroenterology. 2000;119:172–80.
Lada O, Benhamou Y, Cahour A, Katlama C, Poynard T, Thibault V. In vitro susceptibility of lamivudine-resistant hepatitis B virus to adefovir and tenofovir. Antivir Ther. 2004;9:353–63.
Van Bömmel F, Wünsche T, Mauss S, Reinke P, Bergk A, Schürmann D, et al. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40:1421–5.
Lim SG, Ng TM, Kung N, Krastev Z, Volfova M, Husa P, et al. A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B. Arch Intern Med. 2006;166:49–56.
Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–16.
Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800–7.
Wong SN, Lok AS. Update on viral hepatitis: 2005. Curr Opin Gastroenterol. 2006;22:241–7.
Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007;133:1445–51.
Pawlotsky JM, Dusheiko G, Hatzakis A, Lau D, Lau G, Liang TJ, et al. Virologic monitoring of hepatitis B virus therapy in clinical trials and practice: recommendations for a standardized approach. Gastroenterology. 2008;134:405–15.
Song le H, Binh VQ, Duy DN, Kun JF, Bock TC, Kremsner PG, et al. Serum cytokine profiles associated with clinical presentation in Vietnamese infected with hepatitis B virus. J Clin Virol. 2003;28:93–103.
Song le H, Binh VQ, Duy DN, Jüliger S, Bock TC, Luty AJ, et al. Mannose-binding lectin gene polymorphisms and hepatitis B virus infection in Vietnamese patients. Mut Res. 2003;522:119–25.
Peters MG, Hann Hw H, Martin P, Heathcote EJ, Buggisch P, Rubin R, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91–101.
Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–51.
Westland C, Delaney W 4th, Yang H, Chen SS, Marcellin P, Hadziyannis S, et al. Hepatitis B virus genotypes and virologic response in 694 patients in phase III studies of adefovir dipivoxil. Gastroenterology. 2003;125:107–16.
Yuen MF, Sablon E, Libbrecht E, Van De Velde H, Wong DK, Fung J, et al. Significance of viral load, core promoter/precore mutations and specific sequences of polymerase gene in HBV-infected patients on 3-year lamivudine treatment. Antivir Ther. 2006;11:779–86.
Chan HL, Wong VW, Tse CH, Chim AM, Chan HY, Wong GL, et al. Early virological suppression is associated with good maintained response to adefovir dipivoxil in lamivudine resistant chronic hepatitis B. Aliment Pharmacol Ther. 2007;25:891–8.
Werle B, Cinquin K, Marcellin P, Pol S, Maynard M, Trépo C, et al. Evolution of hepatitis B viral load and viral genome sequence during adefovir dipivoxil therapy. J Viral Hepat. 2004;11:74–83.
Hass HG, Bock T, Nehls O, Kaiser S. Early viral kinetics is a strong prognostic predictor for long-term response and occurence of YMDD mutations in patients treated with lamivudine for chronic Hepatitis B. Liver. (submitted).
Lau GK, Cooksley H, Ribeiro RM, Powers KA, Shudo E, Bowden S, et al. Impact of early viral kinetics on T-cell reactivity during antiviral therapy in chronic hepatitis B. Antivir Ther. 2007;12:705–18.
Tillmann HL. Antiviral therapy and resistance with hepatitis B virus infection. World J Gastroenterol. 2007;13:125–40.
Chen CH, Wang JH, Lee CM, Hung CH, Hu TH, Wang JC, et al. Virological response and incidence of adefovir resistance in lamivudine-resistant patients treated with adefovir dipivoxil. Antivir Ther. 2006;11:771–8.
Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK, Kim JH, et al. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006;55:1488–95.
Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385–91.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hass, H.G., Bock, T., Nehls, O. et al. Rapid HBV DNA decrease (week 12) is an important prognostic factor for first-line treatment with adefovir dipivoxil for chronic hepatitis B. J Gastroenterol 44, 871–877 (2009). https://doi.org/10.1007/s00535-009-0078-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-009-0078-y