Abstract
Background
Rheumatoid arthritis (RA) patients are at increased risk of peptic ulcers (PU) induced by nonsteroidal antiinflammatory drugs (NSAIDs). However, the impact of potential drug interactions on the development of PU has yet to be determined in a daily clinical setting. The aim was to estimate the clinical important interactions for PU presented by comedication in Japanese RA outpatients on long-term NSAID treatment.
Methods
This retrospective cohort study enrolled 196 consecutive RA outpatients on NSAID medication for at least 3 months. Potential risk factors for endoscopic PU were analyzed in RA outpatients on longterm NSAID treatment.
Results
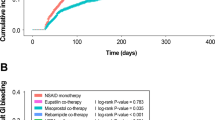
PU incidence was 31% with bisphosphonate co-therapy and 17% without the co-therapy. PU incidence was only 5% in subjects with proton pump inhibitors (PPI) or prostaglandin E1 analogues (PG) co-therapy, 14% with histamine-H2 receptor antagonists(H2RA) co-therapy, and 27% without anti-ulcer agents. In multivariate logistic regression analysis, bisphosphonate co-therapy remained a significant risk factor for PU (OR, 2.29; 95% CI, 1.09–4.81). Other risk factors for ulcer development were advanced age (greater than 60 years) and smoking (OR, 2.58; 95% CI, 1.03–6.49 and OR, 2.71; 95% CI, 1.13–5.53, respectively.) Factors that significantly reduced the incidence of PU were H2RA or PPI/PG cotherapies (OR, 0.29; 95% CI, 0.12–0.68.).
Conclusions
Bisphosphonate co-therapy as well as advanced age and smoking was found to be a significant risk factor in PU, while co-therapies of standard-dose H2RA or PPI/PG proved effective in preventing PU in Japanese RA patients on long-term NSAID treatment.
Similar content being viewed by others
References
Agrawal NM, Van Kerckhove HE, Erhardt LJ, Geis GS. Misoprostol coadministered with diclofenac for prevention of gastroduodenal ulcers. A one-year study. Dig Dis Sci 1995;40:1125–1131.
Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of non-steroidal anti-inflammatory drugs. N Engl J Med 1999;340:1888–1899.
Griffin MR, Piper JM, Daugherty JR, Snowden M, Ray WA. Nonsteroidal anti-inflammatory drug use and increased risk for peptic ulcer disease in elderly persons. Ann Intern Med 1991;114:257–263.
Langman MJ, Weil J, Wainwright P, Lawson DH, Rawlins MD, Logan RF et al. Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994;343:1075–1078.
Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994;343:769–772.
Hallas J, Lauritsen J, Vdladsen HD, Gram LE. Nonsteroidal antiinflammatory drugs and upper gastrointestinal bleeding, identifying high risk groups by excess risk estimates. Scand J Gastroenterol 1995;30:438–444.
Silverstein FE, Graham DY, Senior JR, Davies HW, Struthers BJ, Bittman RM, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, doubleblind, placebo-controlled trial. Ann Intern Med 1995;123:241–249.
Hochain P, Berkelmans I, Czernichow P, Duhamel C, Tranvouez JL, Lerebours E, et al. Which patients taking non-aspirin nonsteroidal anti-inflammatory drugs bleed? A case-control study. Eur J Gastroenterol Hepatol 1995;7:419–426.
Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer disease: role of nonsteroidal antiinflammatory drugs. Ann Intern Med 1991;114:735–740.
Shorr RI, Ray WA, Daugherty JR, Griffin MR. Concurrent use of nonsteroidal anti-inflammatory drugs and oral anticoagulants places elderly persons at high risk for hemorrhagic peptic ulcer disease. Arch Intern Med 1993;153:1665–1670.
Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am J Med 1998;105:31S–38S.
Singh G, Rosen Ramey D. NSAID induced gastrointestinal complications: the ARAMIS perspective-1997. Arthritis, Rheumatism, and Aging Medical Information System. J Rheumatol Suppl 1998;51:8–16.
Shiokawa Y, Nobunaga M, Saito T, Asaki S, Ogawa N. Epidemiology study on upper gastrointestinal lesions induced by nonsteroidal anti-inflammatory drugs. Ryumachi 1991;31:96–111.
Fleisch H. New bisphosphonates in osteoporosis. Osteoporos Int 1993;3:S15–22.
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348:1535–1541.
Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995;333:1437–1443.
Hosking D, Chilvers CE, Christiansen C, Ravn P, Wasnich R, Ross P, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med 1998;338:485–492.
Fitton A, McTavish D. Pamidronate. A review of its pharmacological properties and therapeutic efficacy in resorptive bone disease. Drugs 1991;41:289–318.
Peter CP, Kindt MV, Majka JA. Comparative study of potential for bisphosphonates to damage gastric mucosa of rats. Dig Dis Sci 1998;43:1009–1015.
Kaye PS. Gastric hemorrhage and ulceration in hiatal hernia sac associated with alendronate. Dig Dis Sci 1999;44:903–904.
Blank MA, Gibson GW, Myers WR, Dierckman TA, Phipps RJ, Smith PN. Gastric damage in the rat with nitrogen-containing bisphosphonates depends on pH. Aliment Pharmacol Ther 2000;14:1215–1223.
Lanza FL, Hunt RH, Thomson AB, Provenza JM, Blank MA. Endoscopic comparison of esophageal and gastroduodenal effects of risedronate and alendronate in postmenopausal women. Gastroenterology 2000;119:631–638.
Lowe CE, Depew WT, Vanner SJ, Paterson WG, Meddings JB. Upper gastrointestinal toxicity of alendronate. Am J Gastroenterol 2000;95:634–640.
Lanza F, Schwartz H, Sahba B, Malaty HM, Musliner T, Reyes R, et al. An endoscopic comparison of the effects of alendronate and risedronate on upper gastrointestinal mucosae. Am J Gastroenterol 2000;95:3112–3117.
Lanza F, Rack MF, Simon TJ, Lombardi A, Reyes R, Suryawanshi S. Effects of alendronate on gastric and duodenal mucosa. Am J Gastroenterol 1998;93:753–757.
Graham DY, Malaty HM, Goodgame R. Primary aminobisphosphonates: a new class of gastrotoxic drugs. Comparison of alendronate and aspirin. Am J Gastroenterol 1997;92:1322–1325.
Graham DY, Malaty HM. Alendronate gastric ulcers. Aliment Pharmacol Ther 1999;13:515–519.
Graham DY, Malaty HM. Alendronate and naproxen are synergistic for development of gastric ulcers. Arch Intern Med 2001;161:107–110.
Marshall JK, Rainsford KD, James C, Hunt RH. A randomized controlled trial to assess alendronate-associated injury of the upper gastrointestinal tract. Aliment Pharmacol Ther 2000;14:1451–1457.
Black DM, Cummings SR, Karpf DB. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348:1535–1541.
Bauer DC, Black D, Ensrud K, Thompson D, Hochberg M, Nevitt M, et al. Upper gastrointestinal tract safety profile of alendronate: the fracture intervention trial. Arch Intern Med 2000;160:517–525.
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999;282:1344–1352.
Adami S, Pavelka K, Cline GA, Hosterman MA, Barton IP, Cohen SB, et al. Upper gastrointestinal tract safety of daily oral risedronate in patients taking NSAIDs: a randomized, doubleblind, placebo-controlled trial. Mayo Clin Proc 2005;80:1278–1285.
Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, et al. Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab 2000;85:3109–3115.
Lanza F, Sahba B, Schwartz H, Winograd S, Torosis J, Quan H, et al. The upper GI safety and tolerability of oral alendronate at a dose of 70 milligrams once weekly: a placebo-controlled endoscopy study. Am J Gastroenterol 2002;97:58–64.
Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging 1999;15:15–28.
Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 1998;339:292–299.
Martens MG. Risk of fracture and treatment to prevent osteoporosis-related fracture in postmenopausal women. A review. J Reprod Med 2003;48:425–434.
Lowe CE, Depew WT, Vanner SJ, Paterson WG, Meddings JB. Upper gastrointestinal toxicity of alendronate. Am J Gastroenterol 2000;95:634–634.
Graham DY. What the gastroenterologist should know about the gastrointestinal safety profiles of bisphosphonates. Dig Dis Sci 2002;47:1665–1678.
Lanza F, Rack MF, Simon TJ, Lombardi A, Reyes R, Suryawanshi S. Effects of alendronate on gastric and duodenal mucosa. Am J Gastroenterol 1998;93:753–757.
Lichtenberger LM, Romero JJ, Gibson GW, Blank MA. Effect of bisphosphonates on surface hydrophobicity and phosphatidylcholine concentration of rodent gastric mucosa. Dig Dis Sci 2000;45:1792–1801.
Lichtenberger LM, Wang ZM, Romero JJ, Ulloa C, Perez JC, Giraud MN, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat Med 1995;1:154–158.
Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev 2002;CD002296.
Hooper L, Brown TJ, Elliott R, Payne K, Roberts C, Symmons D. The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review. BMJ 2004;329:948–949.
el-Omar EM, Penman ID, Ardill JE, Chittajallu RS, Howie C, McColl KE. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology 1995;109:681–691.
Feldman M, Richardson CT, Lam SK, Samloff IM. Comparison of gastric acid secretion rates and serum pepsinogen I and II concentrations in Occidental and Oriental duodenal ulcer patients. Gastroenterology 1988;95:630–635.
Blank MA, Gibson GW, Myers WR, Dierckman TA, Phipps RJ, Smith PN. Gastric damage in the rat with nitrogen-containing bisphosphonates depends on pH. Aliment Pharmacol Ther 2000;14:1215–1223.
Eastwood GL. Is smoking still important in the pathogenesis of peptic ulcer disease? J Clin Gastroenterol 1997;25:S1–7.
Ma L, Chow JY, Cho CH. Effects of cigarette smoking on gastric ulcer formation and healing: possible mechanisms of action. J Clin Gastroenterol 1998;27:S80–86.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miyake, K., Kusunoki, M., Shinji, Y. et al. Bisphosphonate increases risk of gastroduodenal ulcer in rheumatoid arthritis patients on long-term nonsteroidal antiinflammatory drug therapy. J Gastroenterol 44, 113–120 (2009). https://doi.org/10.1007/s00535-008-2278-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-008-2278-2