Abstract
Background
Some strains of lactobacilli stimulate immune cells, yet little is known about their potency in cancer prevention. We have previously reported that Lactobacillus casei Shirota (LcS) suppresses murine tumorigenesis through immune modulation. In this study, differences were compared among six representative strains of lactobacilli in regard to their ability to stimulate bone marrow cell-derived dendritic cells (BMDCs) in vitro and tumor suppression in vivo.
Methods
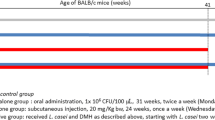
BM-DCs were cocultured with a Lactobacillus strain in vitro, and the interleukin (IL)-12 released into the culture supernatant was measured by enzyme-linked immunosorbent assay. Tumors were chemically induced by a single subcutaneous injection of 3-methylcholanthrene (MC) in BALB/c mice. The test diets containing Lactobacillus were given from the day of the MC injection, and the tumor incidences were monitored. Peyer’s patches were dissected from Lactobacillus-fed mice, and the status of c-Src, a regulator of DCs, in Peyer’s patch cells was examined by Western blotting.
Results
In the coculture system, L. fermentum FERM P-13857 and LcS potently elicited IL-12 production. LcS but not the other strains of lactobacilli showed tumor suppression. The inactive form of c-Src, phosphorylated at Tyr527, was dominantly detected in Peyer’s patches resected from L. fermentum FERM P-13857-fed mice compared with LcS-fed mice.
Conclusions
The responses of DCs may be associated with tumor suppression by an ingested Lactobacillus strain.
Similar content being viewed by others
References
Falk P. Exploring the molecular basis of host-microbial interactions in the GI tract. Biosci Microflora 2002;21:83–97.
Metchinikoff E. The Prolongation of life. 1st ed. New York: G.P. Putnam and Sons; 1908.
Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science 1965;47:747–748.
Fuller R. Probiotics in man and animals. J Appl Bacteriol 1989;66:365–378.
Hirayama K, Rafter J. The role of probiotic bacteria in cancer prevention. Microbes Infect 2000;2:681–686.
Kakizoe T. Asian studies of cancer chemoprevention: latest clinical results. Eur J Cancer 2000;36:1303–1309.
Ishikawa H, Akedo I, Otani T, Suzuki T, Nakamura T, Takeyama I, et al. Randomized trial of dietary fiber and Lactobacillus casei administration for prevention of colorectal tumors. Int J Cancer 2005;116:762–767.
Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis 2001;22:599–605.
Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Inhibitory effect of oral administration of Lactobacillus casei on 3-methylcholanthrene-induced carcinogenesis. Med Microbiol Immunol 1999;188:111–116.
Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, et al. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol 2003;10:259–266.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245.
Rescigno M, Granucci F, Citterio S, Foti M, Ricciardi-Castagnoli P. Coordinated events during bacteria-induced DC maturation. Immunol Today 1999;20:200–203.
Nagoshi M, Sadanaga N, Joo HG, Goedegebuure PS, Eberlein TJ. Tumor-specific cytokine release by donor T cells induces an effective host anti-tumor response through recruitment of host naive antigen presenting cells. Int J Cancer 1999;80:308–314.
Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000;356:1795–1799.
Ohteki T, Fukao T, Suzue K, Maki C, Ito M, Nakamura M, et al. Interleukin 12-dependent interferon gamma production by CD8alpha+ lymphoid dendritic cells. J Exp Med 1999;189:1981–1986.
Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 1999;5:405–411.
Nagao F, Nakayama M, Muto T, Okumura K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the immune system in healthy human subjects. Biosci Biotechnol Biochem 2000;64:2706–2708.
Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/ macrophage colony-stimulating factor. J Exp Med 1992;176:1693–1702.
Sato M, Iwakabe K, Kimura S, Nishimura T. Functional skewing of bone marrow-derived dendritic cells by Th1-or Th2-inducing cytokines. Immunol Lett 1999;67:63–68.
Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today 1999;20:561–567.
Arron JR, Vologodskaia M, Wong BR, Naramura M, Kim N, Gu H, et al. A positive regulatory role for Cbl family proteins in tumor necrosis factor-related activation-induced cytokine (trance) and CD40L-mediated Akt activation. J Biol Chem 2001;276:30011–30017.
Aki D, Mashima R, Saeki K, Minoda Y, Yamauchi M, Yoshimura A. Modulation of TLR signalling by the C-terminal Src kinase (Csk) in macrophages. Genes Cells 2005;10:357–368.
Hunter T. A tail of two src’s: mutatis mutandis. Cell 1987;49:1–4.
Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol 1996;26:659–668.
Kronin V, Hochrein H, Shortman K, Kelso A. Regulation of T cell cytokine production by dendritic cells. Immunol Cell Biol 2000;78:214–223.
Gabrilovich DI, Nadaf S, Corak J, Berzofsky JA, Carbone DP. Dendritic cells in anti-tumor immune responses. II. Dendritic cells grown from bone marrow precursors, but not mature DC from tumor-bearing mice are effective antigen carriers in the therapy of established tumors. Cell Immunol 1996;170:111–119.
Chaux P, Favre N, Martin M, Martin F. Tumor-infiltrating dendritic cells are defective in their antigen-presenting function and inducible B7 expression in rats. Int J Cancer 1997;72:619–624.
Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res 1997;3:483–490.
Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 2001;166:678–689.
Noguchi Y, Jungbluth A, Richards EC, Old LJ. Effect of interleukin 12 on tumor induction by 3-methylcholanthrene. Proc Natl Acad Sci USA 1996;93:11798–11801.
Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol 2002;168:171–178.
Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA 2005;102:2880–2885.
Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of probiotics. J Nutr 1995;125:1401–1412.
Inohara N, Ogura Y, Chen FF, Muto A, Nunez G. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem 2001;276:2551–2554.
Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol 2003;4:702–707.
Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol 2003;23:7531–7539.
Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA 1998;95:588–593.
Poltorak A, He X, Smirnova I, Liu MY, van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998;282:2085–2088.
Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 1999;11:443–451.
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 2004;118:229–241.
Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science 2000;289:1560–1563.
Matsumoto S, Hara T, Hori T, Mitsuyama K, Nagaoka M, Tomiyasu N, et al. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol 2005;140:417–426.
Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci USA 2004;101:6110–6115.
Takahashi M, Iwata S, Yamazaki N, Fujiwara H. Phagocytosis of the lactic acid bacteria by M cells in the rabbit Peyer’s patches. J Clin Electron Microsc 1991;24:5–6.
MacDonald TT, Monteleone G. IL-12 and Th1 immune responses in human Peyer’s patches. Trends Immunol 2001;22:244–247.
Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001;2:361–367.
Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005;307:254–258.
Abu-Amer Y, Ross FP, Edwards J, Teitelbaum SL. Lipopolysac-charide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J Clin Invest 1997;100:1557–1565.
Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell 1999;4:1041–1049.
Napolitani G, Bortoletto N, Racioppi L, Lanzavecchia A, D’Oro U. Activation of src-family tyrosine kinases by LPS regulates cytokine production in dendritic cells by controlling AP-1 formation. Eur J Immunol 2003;33:2832–2841.
Galgani M, de Rosa V, de Simone S, Leonardi A, D’Oro U, Napolitani G, et al. Cyclic AMP modulates the functional plasticity of immature dendritic cells by inhibiting Src-like kinases through protein kinase A-mediated signaling. J Biol Chem 2004;279:32507–32514.
Setoyama T, Nomoto K, Yokokura T, Mutai M. Protective effect of lipoteichoic acid from Lactobacillus casei and Lactobacillus fermentum against Pseudomonas aeruginosa in mice. J Gen Microbiol 1985;131:2501–2503.
Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 2006;116:1310–1316.
Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol 2006;7:929–936.
Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 2006;7:937–945.
Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi, et al. Potent antitumor activity of interleukin-27. Cancer Res 2004;64:1152–1156.
Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med 2006;12:693–698.
Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007;450:903–907.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takagi, A., Ikemura, H., Matsuzaki, T. et al. Relationship between the in vitro response of dendritic cells to Lactobacillus and prevention of tumorigenesis in the mouse. J Gastroenterol 43, 661–669 (2008). https://doi.org/10.1007/s00535-008-2212-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-008-2212-7