Abstract
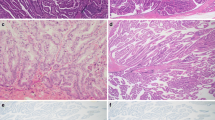
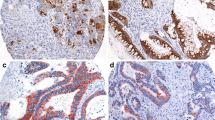
Mucins are high molecular weight glycoproteins that play important roles in carcinogenesis and tumor invasion. We have described, for the first time, that pancreatic ductal adenocarcinomas (PDACs) with an aggressive behavior and a poor outcome expressed MUC1 (pan-epithelial membrane-associated mucin) but did not express MUC2 (intestinal-type secreted mucin), whereas intraductal papillary mucinous neoplasms (IPMNs) with indolent behavior and a favorable outcome did not express MUC1 but did express MUC2. These expression profiles of MUC1 and MUC2 related to the prognoses of the patients were also observed in biliary neoplasms such as intrahepatic cholangiocarcinoma (ICC)-mass-forming type (MF), mucin-producing bile duct tumor (MPBT), and extrahepatic bile duct carcinoma (EHBDC). We also found recently that high expression of MUC4 (tracheobronchial membrane-associated mucin) in PDACs, ICCs-MF, and EHBDCs was a new independent poor prognostic factor, although MUC4 was not expressed in normal pancreatobiliary tissue. High de novo expression of MUC5AC (gastric-type secreted mucin) was observed in many types of pancreatobiliary neoplasms, including all grades of pancreatic intraepithelial neoplasia (PanIN) and biliary intraepithelial neoplasia (BilIN), and all types of IPMNs and MPBTs, as well as PDACs and ICCs-MF, although MUC5AC was not expressed in normal pancreatobiliary tissue. The combined status of MUC1, MUC2, MUC4, and MUC5AC expression may be useful for the early detection of pancreatobiliary neoplasms and evaluation of their malignancy. In regard to the mechanism of mucin expression, we have recently reported that MUC1, MUC2, MUC4, and MUC5AC gene expression is regulated by epigenetics (DNA methylation and histone H3 lysine 9 modification) in cancer cell lines, including PDAC cells. Translational research of mucin gene expression mechanisms, including epigenetics, in pancreatobiliary neoplasms may give us new tools for the early and accurate detection of these neoplasms.














Similar content being viewed by others
References
Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909.
Isaji S, Kawarada Y, Uemoto S. Classification of pancreatic cancer: comparison of Japanese and UICC classifications. Pancreas. 2004;28:231–4.
Ishikawa O, Ohigashi H, Imaoka S, Nakaizumi A, Uehara H, Kitamura T, et al. Minute carcinoma of the pancreas measuring 1 cm or less in diameter—collective review of Japanese case reports. Hepatogastroenterology. 1999;46:8–15.
Egawa S, Takeda K, Fukuyama S, Motoi F, Sunamura M, Matsuno S. Clinicopathological aspects of small pancreatic cancer. Pancreas. 2004;28:235–40.
Inoue K, Makuuchi M, Takayama T, Torzilli G, Yamamoto J, Shimada K, et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery. 2000;127:498–505.
Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002;89:1525–31.
Wade TP, Prasad CN, Virgo KS, Johnson FE. Experience with distal bile duct cancers in US. Veterans affairs hospitals: 1987–1991. J Surg Oncol. 1997;64:242–5.
Fong Y, Blumgart LH, Lin E, Fortner JG, Brennan MF. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996;83:1712–5.
Ouchi K, Matsuno S, Sato T. Long-term survival in carcinoma of the biliary tract. Analysis of prognostic factors in 146 resections. Arch Surg. 1989;124:248–52.
Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system: 8 tumours of the liver and intrahepatic bile ducts, p. 157–99; 10 Tumours of the exocrine pancreas, p. 219–50. Lyon: IARC; 2000.
Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, et al. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary–mucinous neoplasms. Am J Surg Pathol. 2004;28:327–38.
Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333–43.
Takaori K. Current understanding of precursors to pancreatic cancer. J Hepatobiliary Pancreat Surg. 2007;14:217–23.
Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–86.
Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87.
Zen Y, Adsay NV, Bardadin K, Colombari R, Ferrell L, Haga H, et al. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol. 2007;20:701–9.
Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surfaces. Nat Rev Cancer. 2004;4:45–60.
Yonezawa S, Sato E. Expression of mucin antigens in human cancers and its relationship with malignancy potential. Pathol Int. 1997;47:813–30.
Yonezawa S, Goto M, Yamada N, Higashi M, Nomoto M. Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics. 2008;8:3329–41.
Lehmann JM, Riethmuller G, Johnson JP. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci USA. 1989;86:9891–5.
Chen Y, Zhao YH, Kalaslavadi TB, Hamati E, Nehrke K, Le AD, et al. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol. 2004;30:155–65.
Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J Biol Chem. 2004;279:1968–79.
Itoh Y, Kamata-Sakurai M, Denda-Nagai K, Nagai S, Tsuiji M, Ishii-Schrade K, et al. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18:74–83.
Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192–206.
Osako M, Yonezawa S, Siddiki B, Huang J, Ho JJ, Kim YS, et al. Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer. 1993;71:2191–9.
Yamashita K, Yonezawa S, Tanaka S, Shirahama H, Sakoda K, Imai K, et al. Immunohistochemical study of mucin carbohydrates and core proteins in hepatolithiasis and cholangiocarcinoma. Int J Cancer. 1993;55:82–91.
Kitamura H, Yonezawa S, Tanaka S, Kim YS, Sato E. Expression of mucin carbohydrates and core proteins in carcinomas of the ampulla of Vater: their relationship to prognosis. Jpn J Cancer Res. 1996;87:631–40.
Yonezawa S, Sueyoshi K, Nomoto M, Kitamura H, Nagata K, Arimura Y, et al. MUC2 gene expression is found in noninvasive tumors but not in invasive tumors of the pancreas and liver: its close relationship with prognosis of the patients. Hum Pathol. 1997;28:344–52.
Higashi M, Yonezawa S, Ho JJ, Tanaka S, Irimura T, Kim YS, et al. Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinoma. Hepatology. 1999;30:1347–55.
Yonezawa S, Nakamura A, Horinouchi M, Sato E. The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg. 2002;9:328–41.
Nakamura A, Horinouchi M, Goto M, Nagata K, Sakoda K, Takao S, et al. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol. 2002;197:201–10.
Horinouchi M, Nagata K, Nakamura A, Goto M, Takao S, Sakamoto M, et al. Expression of different glycoforms of membrane mucin (MUC1) and secretory mucin (MUC2, MUC5AC and MUC6) in pancreatic neoplasms. Acta Histochem Cytochem. 2003;36:443–53.
Tamada S, Goto M, Nomoto M, Nagata K, Shimizu T, Tanaka S, et al. Expression of MUC1 and MUC2 mucins in extrahepatic bile duct carcinomas: its relationship with tumor progression and prognosis. Pathol Int. 2002;52:713–23.
Yonezawa S, Higashi M, Yamada N, Goto M. Precursor lesions of pancreatic cancer. Gut and Liver. 2008;2:137–54.
Carraway KL, Ramsauer VP, Haq B, Carothers Carraway CA. Cell signaling through membrane mucins. Bioessays. 2003;25:66–71.
Shibahara H, Tamada S, Higashi M, Goto M, Batra SK, Hollingsworth MA, et al. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39:220–9.
Saitou M, Goto M, Horinouchi M, Tamada S, Nagata K, Hamada T, et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845–52.
Tamada S, Shibahara H, Higashi M, Goto M, Batra SK, Imai K, et al. MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clin Cancer Res. 2006;12:4257–64.
Nagata K, Horinouchi M, Saitou M, Higashi M, Nomoto M, Goto M, et al. Mucins expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243–54.
Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, et al. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052–60.
Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, et al. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct—an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006;44:249–50.
Yamada N, Nishida Y, Tsutsumida H, Hamada T, Goto M, Higashi M, et al. MUC1 expression is regulated by DNA methylation and histone H3 lysine 9 modification in cancer cells. Cancer Res. 2008;68:2708–16.
Hamada T, Goto M, Tsutsumida H, Nomoto M, Higashi M, Sugai T, et al. Mapping of the methylation pattern of the MUC2 promoter in pancreatic cancer cell lines, using bisulfite genomic sequencing. Cancer Lett. 2005;227:175–84.
Yamada N, Hamada T, Goto M, Tsutsumida H, Higashi M, Nomoto M, et al. MUC2 expression is regulated by histone H3 modification and DNA methylation in pancreatic cancer. Int J Cancer. 2006;119:1850–7.
Yamada N, Nishida Y, Tsutsumida H, Goto M, Higashi M, Nomoto M, et al. Promoter CpG methylation in cancer cells contributes to regulation of MUC4. Br J Cancer. 2009;100:344–51.
Yamada N, Yokoyama S, Nishida Y, Houjou I, Tsutsumida H, Goto M, et al. CpG methylation in the distal region of MUC5AC promoter contributes to the regulation of MUC5AC. Abstract in 68th annual meeting of the Japanese Cancer Society, Yokohama, 1–3 Oct 2009.
Swartz MJ, Batra SK, Varshney GC, Hollingsworth MA, Yeo CJ, Cameron JL, et al. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791–6.
Park HU, Kim JW, Kim GE, Bae HI, Crawley SC, Yang SC, et al. Aberrant expression of MUC3 and MUC4 membrane-associated mucins and sialyl Le(x) antigen in pancreatic intraepithelial neoplasia. Pancreas. 2003;26:e48–54.
Lüttges J, Zamboni G, Longnecker D, Klöppel G. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol. 2001;25:942–8.
Yonezawa S, Horinouchi M, Osako M, Kubo M, Takao S, Arimura Y, et al. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int. 1999;49:45–54.
Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–48.
Furukawa T, Klöppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–9.
Terris B, Dubois S, Buisine MP, Sauvanet A, Ruszniewski P, Aubert JP, et al. Mucin gene expression in intraductal papillary-mucinous pancreatic tumours and related lesions. J Pathol. 2002;197:632–7.
Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32.
Adsay NV, Merati K, Andea A, Sarkar F, Hruban RH, Wilentz RE, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–95.
Yonezawa S, Taira M, Osako M, Kubo M, Tanaka S, Sakoda K, et al. MUC-1 mucin expression in invasive areas of intraductal papillary mucinous tumors of the pancreas. Pathol Int. 1998;48:319–22.
Gum JR Jr. Mucin genes and the proteins they encode: structure, diversity, and regulation. Am J Respir Cell Mol Biol. 1992;7:557–64.
Gum JR Jr, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994;269:2440–6.
Kim YS, Gum JR Jr. Diversity of mucin genes, structure, function, and expression. Gastroenterology. 1995;109:999–1001.
Pigny P, Guyonnet-Duperat V, Hill AS, Pratt WS, Galiegue-Zouitina S, d’Hooge MC, et al. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics. 1996;38:340–52.
Buisine MP, Janin A, Maunoury V, Audié JP, Delescaut MP, Copin MC, et al. Aberrant expression of a human mucin gene (MUC5AC) in rectosigmoid villous adenoma. Gastroenterology. 1996;110:84–91.
Lüttges J, Feyerabend B, Buchelt T, Pacena M, Klöppel G. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 2002;26:466–71.
Terada T, Ohta T, Sasaki M, Nakanuma Y, Kim YS. Expression of MUC apomucins in normal pancreas and pancreatic tumours. J Pathol. 1996;180:160–5.
Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–22.
Nagata K. Analysis of mucins and CD10 expression in pancreatic intraductal neoplasia (in Japanese, with English abstract). Kagoshimadaigaku Igakuzashi (Med J Kagoshima Univ). 2005;57:7–17.
Liver Cancer study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer, the 5th edition, revised version. Tokyo: Kanehara; 2009.
Kim HJ, Kim MH, Lee SK, Yoo KS, Park ET, Lim BC, et al. Mucin-hypersecreting bile duct tumor characterized by a striking homology with an intraductal papillary mucinous tumor (IPMT) of the pancreas. Endoscopy. 2002;32:389–93.
Klöppel G, Kosmahl M. Is the intraductal papillary mucinous neoplasia of the biliary tract a counterpart of pancreatic papillary mucinous neoplasm? J Hepatol. 2006;44:249–50.
Sasaki M, Nakanuma Y, Kim YS. Expression of apomucins in the intrahepatic biliary tree in hepatolithiasis differs from that in normal liver and extrahepatic biliary obstruction. Hepatology. 1998;27:54–61.
Sakamoto E, Hayakawa N, Kamiya J, Kondo S, Nagino M, Kanai M, et al. Treatment strategy for mucin-producing intrahepatic cholangiocarcinoma: value of percutaneous transhepatic biliary drainage and cholangioscopy. World J Surg. 1999;23:1038–43. discussion 1043–4.
Sakamoto E, Hayakawa N, Kamiya J, Kondo S, Nagino M, Kanai M, et al. Clinicopathological studies of mucin-producing cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 1997;4:157–62.
Adsay NV, Conlon KC, Zee SY, Brennan MF, Klimstra DS. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77.
Ringel J, Lohr M. The MUC gene family: their role in diagnosis and early detection of pancreatic cancer. Mol Cancer. 2003;2:1–5.
Kim YS, Gum JR Jr, Crawley SC, Deng G, Ho JJ. Mucin gene and antigen expression in biliopancreatic carcinogenesis. Ann Oncol. 1999;10(Suppl 4):51–5.
Jalanko H, Kuusela P, Roberts P, Sipponen P, Haglund CA, Makela O. Comparison of a new tumour marker, CA19-9, with alpha-fetoprotein and carcinoembryonic antigen in the patients with upper gastrointestinal diseases. J Clin Pathol. 1984;37:218–22.
Kuusela P, Jalanko H, Roberts P, Sipponen P, Mecklin JP, Pitkänen R, et al. Comparison of CA19–9 and carcinoembryonic antigen (CEA) levels in the serum of patients with colorectal diseases. Br J Cancer. 1984;49:135–9.
Ohuchida K, Mizumoto K, Yamada D, Fujii K, Ishikawa N, Konomi H, et al. Quantitative analysis of MUC1 and MUC5AC mRNA in pancreatic juice for preoperative diagnosis of pancreatic cancer. Int J Cancer. 2006;118:405–11.
Matsuno YK, Saito T, Gotoh M, Narimatsu H, Kameyama A. Supported molecular matrix electrophoresis: a new tool for characterization of glycoproteins. Anal Chem. 2009;81:3816–23.
Siddiqui J, Abe M, Hayes D, Shani E, Yunis E, Kufe D. Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci USA. 1988;85:2320–23.
Shibahara F, Goto M, Nimura Y, Yonezawa S. Pathological features of mucin-producing bile duct tumors: comparison with pancreatic intraductal papillary-mucinous neoplasms (in Japanese with English abstract). Gazou Shindan. 2006;26:595–602.
Shibahara F, Yonezawa S, Nimura Y. Mucin-producing bile duct tumors with comparison to pancreatic IPMN in expression of mucin protein (in Japanese). Tan to Sui. 2006;27:451–7.
Acknowledgments
We thank Ms. Eri Minamida and Ms. Mariko Tanaka for their secretarial assistance. This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Scientific Research on Priority Areas 20014022 and Scientific Research (C) 20590345 to S. Yonezawa, and Scientific Research (C) 21590399 to M. Higashi).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yonezawa, S., Higashi, M., Yamada, N. et al. Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Sci 17, 108–124 (2010). https://doi.org/10.1007/s00534-009-0174-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-009-0174-7