Abstract
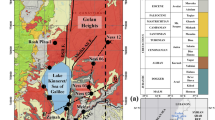
Conjoint consideration of distribution of major, rare earth elements (REE) and Y (combined to REY) and of H, O, C, S, Sr isotopes reveals that four types of groundwater are distinguishable by their chemical composition presented by spider patterns. REY patterns indicate thermo-saline deep water and two types of shallow saline groundwaters. Presence of connate waters is not detectable. Sr isotope ratios distinguish three sources of Sr: fast and slow weathering of biotite and K-feldspar in Pleistocene sediments, respectively, and dissolution of limestones. δ13C(DIC) indicate dissolution of limestone under closed and open system conditions. Numerous samples show δ13C(DIC) > 13‰ which is probably caused by incongruent dissolution of calcite and dolomite. The brines from below 1,000 m represent mixtures of pre-Pleistocene seawater or its evaporation brines and infiltrated post-Pleistocene precipitation. The shallow waters represent mixtures of Pleistocene and Recent precipitation salinized by dissolution of evaporites or by mixing with ascending brines. The distribution of water types is independent on geologic units and lithologies. Even the Tertiary Rupelian aquiclude does not prevent salinization of the upper aquifer.













Similar content being viewed by others
References
Anders E, Grevesse N (1989) Abundance of elements: meteoric and solar. Geochim Cosmochim Acta 53:197–214
Bau M (1999) Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: experimental evidence for Ce oxidation, Y–Ho fractionation, and lanthanide tetrad effect. Geochim Cosmochim Acta 63:67–77
Bau M, Dulski P (1996) Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet Sci Lett 143:245–255
Bausch WM (1968) Outlines of distribution of strontium in marine limestones. In: Müller G, Friedman GM (eds) Carbonate sedimentology in Central Europe. Springer, Berlin, pp 106–115
Bein A, Dutton AR (1993) Origin, distribution, and movement of brine in the Permian Basin (USA): a model for displacement of connate water. Geol Soc Am Bull 105:695–707
Bennett SS, Hanor JS (1987) Dynamics of subsurface salt dissolution at the Welsh Dome, Lousiana Gulf Coast. In: Lerch I, O’Brien JJ (eds) Dynamical geology of salt and related structures. Academic, Orlando, pp 653–677
Berner ZA, Stüben D, Leosson MA, Kling H (2002) S- and O isotopic character of dissolved sulphate in the cover rock aquifer of a Zechstein salt dome. Appl Geochem 17:1515–1528
Blum JD, Erel Y (1995) A silicate weathering mechanism linking increase in marine 87Sr/86Sr with global glaciation. Nature 373:415–418
Bruland KW, Lohan MC (2004) Controls of trace metals in seawater. In: Holland HD, Turekian KK (eds) Treatise on geochemistry, vol 6, pp 23–47. Elsevier, Amsterdam
Buckau G, Artinger R, Geyer S, Wolf M, Fritz P, Kim JI (2000) 14C dating of Gorleben groundwater. Appl Geochem 15:583–597
Bullen TD, Kendall C (1998) Tracing of weathering reactions and water flowpaths: a multi-isotopic approach. In: Kendall C, McDonnel JJ (eds) Isotope tracers in catchment hydrology. Elsevier Amsterdam, pp 611–646
Carpenter AB (1978) Origin and chemical evolution of brines in sedimentary basins. Oklahoma Geol Surv Circ 79:60–77
Clark I, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, Boca Raton, pp 1–328
Claypool GE, Holser WT, Kaplan IR, Sakai H, Zak I (1980) The age curves of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem Geol 28:199–260
Clayton RN, Friedmann I, Graf DL, Mayeda TK, Meents WF, Shimp NF (1966) The origin of saline formation waters. J Geophys Res 71:3869–3882
Craig H (1961) Isotopic variations in meteoric waters. Sci 133:1702–1703
Domenico PA, Robbins GA (1985) The displacement of connate water from aquifers. Geol Soc Am Bul 96:328–335
Dulski P (1994) Interferences of oxide, hydroxide and chloride analyte species in the determination of rare earth elements in geological samples by inductively coupled plasma-mass spectrometry. Fresenius J Anal Chem 350:194–203
Farber E, Vengosh A, Gavrieli I, Marie A, Bullen TD, Mayer B, Holtzman R, Segal M, Shavit U (2004) The origin and mechanism of salinization of the Lower Jordan River. Geochim Cosmochim Acta 60:1989–2006
Fontes JC, Matray JM (1993) Geochemistry and origin of formation brines from the Paris basin, France, 1: brines associated with Triassic salts. Chem Geol 109:149–175
Frape SK, Fritz P (1987) Geochemical trends for groundwaters from the Canadian shield. In: Fritz P, Frape SK (eds) Saline water and gases in crystalline rocks. Geol Ass Can Spec Pap 33:19–38
Fritz P, Basharmal GM, Drimmie RJ, Ibsen J, Quereshi RM (1989) Oxygen isotope exchange between sulphate and water during bacterial reduction of sulphate. Chem Geol 79:99–105
Giggenbach WF (1992) Isotopic shifts in waters from geothermal and volcanic systems along convergent plate boundaries and their origin. Earth Planet Sci Lett 113:495–510
Graf DL, Meents WF, Friedman I, Shimp NF (1966) The origin of saline formation waters III. Calcium–chloride waters. US Geol Surv Circ 397:1–60
Grube A, Lotz B (2004) Geological and numerical modeling of geogenic salinization in the area of the Lübeck basin (North Germany). Groundwater and saline intrusion. Selected papers from the 18th Salt Water Intrusion Meeting (SWIM), 31 May–3 June, Instituto geológico y minero de España, Cartagena, pp 183–195
Grube A, Nachtigall KH, Wichmann K (1996) Zur Grundwasserversalzung in Schleswig-Holstein. Meyniana 48:21–34
Grube A, Wichman K, Hahn J, Nachtigall KH (2000) Geogene Grundwasserversalzung in den Porengrundwasserleitern Norddeutschlands und ihre Bedeutung für die Wasserwirtschaft. Technologiezentrum Wasser Karlsruhe (TZW), Karlsruhe 9, pp 1–203
Hannemann M, Schirrmeister W (1998) Paläohydrogeologische Grundlagen der Entwicklung der Süß-/Salzwassergrenze und der Salzwasseraustritte in Brandenburg (Paleo-hydrogeological principles of the development of the fresh–/saltwater interface and the occurrence of saline springs in Brandenburg). Brandenburg Geowiss Beitr 5:61–72
Hanor JS (1994) Origin of saline fluids in sedimentary basins. In: Parnell J (ed) Geofluids: origin, migration and evolution of fluids in sedimentary basins. Geol Soc Spec Publ 151–178
Hardie LA (1990) The roles of rifting and hydrothermal CaCl2 in the origin of potash evaporites—an hypothesis. Am J Sci 290:43–106
Herut B, Starinsky A, Katz A, Bein A (1990) The role of seawater freezing in the formation of subsurface brines. Geochim Cosmochim Acta 54:13–21
Jortzig H (2002) Verbreitung der Rupelfolge—Das Geopotenzial Brandenburgs. In: Stackebrandt W, Manhenke V (eds) Atlas zur Geologie von Brandenburg. Landesamt für Geowissenschaften und Rohstoffe Brandenburg, Kleinmachnow, vol 2, pp 72–73
Katz BG, Plummer LN, Busenberg E, Revesz KM, Jones BF, Lee TM (1995) Chemical evolution of groundwater near a sinkhole lake, northern Florida. 2. Chemical patterns, mass transfer modelling, and rates of mass transfer reactions. Water Resour Res 31:1565–1584
Kawabe I, Ohta A, Ishii S, Tokumura M, Miyauchi K (1999) REE partitioning between Fe–Mn oxyhydroxide precipitates and weakly acid NaCl solution: convex tetrad effect and fractionation of Y and Sc from heavy lanthanides. Geochem J 33:167–179
Kharaka YK, Berry FAF (1973) Simultaneous flow of water and solutes through geological membranes: I. experimental investigations. Geochim Cosmochim Acta 37:2577–2603
Klinge H, Vogel P, Schelkes K (1992) Chemical composition and origin of saline formation waters from the Konrad mine, Germany. Water rock interaction, Proceedings of the 7th international symposium on water–rock interaction/WRI-7, Park City, Utah, USA, 13–18 July, Balkema, Rotterdam pp 1117–1120
Kloppmann W, Negrel Ph, Casanova J, Klinge H, Schelke K, Guerrot C (2001) Halite dissolution derived brines in the vicinity of a Permian salt dome (N German Basin). Evidence from boron, strontium, oxygen and hydrogen isotopes. Geochim Cosmochim Acta 65:4087–4101
Knauth LP (1988) Origin and mixing history of brines, Palo Duro Basin, Texas, USA. Appl Geochem 3:455–474
Knöller K, Weise SM (in prep) Utilization of high temperature pyrolysis for the determination of 2H/1H and 18O/16O ratios in high salinity waters
Knöller K, Vogt C, Richnow H-H, Weise SM (2006) Sulfur and oxygen isotope fractionation during benzene, toluene, ethyl benzene, and xylene degradation by sulfate-reducing bacteria. Environ Sci Technol 40:3879–3885
Lerman A (1970) Chemical equilibrium and evolution of chloride brines. 50th Anniv Symp Miner Soc Spec Pap 3:291–306
Magri F, Bayer U, Jahnke C, Clausnitzer V, Diersch HJ, Fuhrmann J, Möller P, Pekdeger A, Tesmer M, Voigt H-J (2005) Fluid dynamics driven saline water in the North East German Basin. Int J Earth Sci 94:1056–1069
McLennan SM (1989) Rare earth elements in sedimentary rocks: influence of provenance and sedimentary processes. In: Lipin BR, McKray GA (eds) Geochemistry and mineralogy of rare earth elements. Mineralogical Society of America, Washington DC, pp 169–200
Metha S, Fryar AE, Banner JL (2000) Controls on the regional-scale salinization of the Ogallala aquifer, Southern High Plains, Texas USA. Appl Geochem 15:849–864
Mills AL (1988) Variation in δ13C of stream bicarbonate: implications for sources of alkalinity. M.S. thesis, George Washington University, Washington DC, p 160
Möller P, Rosenthal E, Dulski P, Geyer S, Guttman Y (2003) Rare earths and yttrium hydrostratigraphy along the Lake Kinneret—Dead Sea—Arava transform fault, Israel. Appl Geochem 18:1613–1628
Möller P, Geyer S, Salameh E, Dulski P (2006a) Sources of mineralization of thermal groundwater of Jordan. Acta Hydrochim Hydrobiol 34:86–100
Möller P, Dulski P, Salameh E, Geyer S (2006b) Characterization of sources of thermal spring- and well water in Jordan by rare earth elements and yttrium distribution and stable isotopes of H2O. Acta Hydrochim Hydrobiol 34:101–116
Möller P, Rosenthal E, Geyer S, Guttman J, Dulski P, Rybakov M, Zilberbrand M, Jahnke C, Flexer A (2007) Hydrochemical processes in the lower Jordan Valley and the Dead Sea area. Chem Geol 239:27–49
Nordstrom DK, Olsson T (1987) Fluid inclusions as a source of dissolved salts in deep granitic groundwaters. In: Fritz P, Frape SK (eds) Saline waters and gases in crystalline rocks. Geol Assoc Can Spec Pap 33:111–119
Quinn KA, Byrne RH, Schijf J (2004) Comparative scavenging of yttrium and the rare earth elements in seawater: competitive influences of solution and surface chemistry. Aquat Chem 100:59–80
Rosenthal E (1997) Thermosaline water of Ca–chloride composition: diagnostics and brine evolution. Environ Geol 32:245–249
Scheck M, Bayer U (1999) Evolution of the Northeast German Basin—inferences from a 3D structural model and subsidence analysis. Tectonophysics 313:145–168
Schidlowski M, Hayes JM, Kaplan IR (1983) Isotopic inferences of ancient biochemistries: carbon, sulphur, hydrogen and nitrogen. In: Schopf JW (ed) Earth’s earliest biosphere: its origin and evolution. Princeton University Press, Princeton, pp 149–186
Sofer Z, Gat JR (1972) Activities and concentrations of oxygen-18 in concentrated aqueous salt solutions: analytical and geophysical implications. Earth Planet Sci Lett 15:232–238
Spencer DW (1987) Ocean chemistry—its central role in the evolution of the Earth. Chem Int 9/5:196–205
Starinsky A (1974) Relationship between Ca–chloride brines and sedimentary rocks in Isreal. Ph.D. thesis, Hebrew University, Jerusalem
Tesmer M, Otto R, Pekdeger A, Möller P, Bayer U, Magri F, Fuhrmann J, Enchery G, Jahnke C, Voigt H-J (2005) Migration paths and hydrochemical processes of groundwater salinization in different aquifer systems of the North German Basin. Terra Nostra 05/0:123–126
Tesmer M, Möller P, Wieland S, Jahnke C, Pekdeger A, Voigt H (2007) Deep reaching fluid flow in the North-East German Basin. Origin and processes of groundwater salinisation. Hydrol J, doi:10.1007/s10040–007-0176-y
Toran L, Harris RF (1989) Interpretation of sulfur and isotopes in biological and abiological sulphide oxidation. Geochim Coschim Acta 53:2341–2348
Van Stempvoort DR, Krouse HR (1994) Controls of δ18O in sulphate—review of experimental data and applications to specific environments. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulphide oxidation. Am Chem Soc Symp Ser 550:447–480
Veizer J, Ala D, Azmy K, Bruckschen P, Buhl D, Bruhn F, Carden GAF, Diener A, Ebneth S, Goddris Y, Jasper T, Korte C, Pawellek F, Podlaha OG, Strauss H (1999) 87Sr/86Sr,(δ13C and δ18O evolution of Phanerozoic seawater. Chem Geol 161:59–88
Wiegand B, Dietzel M, Bielert U, Groth P, Nansen BT (2001) 87Sr/86Sr-Verhältnisse als Tracer für geochemische Prozesse in einem Lockergesteinsaquifer (Liebenau, NW Deutschland). Acta Hdrochim Hydrobiol 29:139–152
Wigley TML, Plummer LN, Pearson FJ (1978) Mass transfer and carbon isotope evolution in natural water systems. Geochim Cosmochim Acta 42:117–1139
Yanagisawa F, Sakai H (1983) Thermal decomposition of barium sulphate–vanadium pentaoxide–silica glass mixtures for preparation of sulfur dioxide in sulfur isotope ratio measurements. Anal Chem 55:985–987
Zieschang J (1974) Ergebnisse und Tendenzen hydrogeologischer Forschungen in der DDR (Results and tendencies of hydrogeological research in the GDR). Zt Angew Geol 20:452–458
Acknowledgments
This study has been funded by the German Science Foundation (DFG) as part of SPP 1135 “Dynamics of sedimentary systems under varying stress conditions by example of the Central European Basin system”. We thank B. Hansen, University of Göttingen, for supplying Sr isotope ratios. The authors appreciated the critical comments of W. Kloppmann and an anonymous reviewer who improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Möller, P., Weise, S.M., Tesmer, M. et al. Salinization of groundwater in the North German Basin: results from conjoint investigation of major, trace element and multi-isotope distribution. Int J Earth Sci (Geol Rundsch) 97, 1057–1073 (2008). https://doi.org/10.1007/s00531-007-0211-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00531-007-0211-1