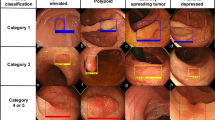
Abstract
Colorectal cancer (CRC) is one of the most lethal kinds of cancer, so early detection is critical. Three datasets, namely CNN transfer learning with discrete wavelet transform (DWT), discrete cosine transform (DCT), and support vector machines (SVMs), were used to find CRC. In these instances, a quick and precise visual diagnosis of polyps is needed in the current scenario. The proposed process involves four distinct phases. First and foremost, convolutional neural networks (CNNs) are developed to test the efficacy of the model. Further, a transfer learning approach was incorporated using SVM and LSTM. Using the K-means technique, a visual explanation is finally presented. This system works with the balanced Hyper Kvasir and mixed datasets, which are made up of CVC Clinic DB, Kvasir2, and Hyper Kvasir. The system is called "ColoRectalCADx". The convolutional neural network (CNN) models are ResNet-50V2, DenseNet-201, VGG-16, and RDV-22. The system achieved the highest accuracy with CNN DesnseNet-201 in Hyper Kvasir (98.92% training, 98.91% testing, 93.62% SVM training, and 95.87% SVM tests). CNN DenseNet-201 also achieved the highest accuracy with the mixed dataset (98.91% training, 96.13% testing, 95.41% SVM training, and 94.86% SVM testing). The process involved three phases, namely individual CNN, combination of CNN with SVM, and combination of CNN, LSTM, and SVM. After three phases of the system, across both datasets, the CNN + SVM + LSTM combination was proven to be the most effective. Finally, the unsupervised K-means learning algorithm extracts the location of any cancerous polyps and upon classification using SVM classifier resulted with an accuracy of 80%. The K-means algorithm, which uses segmented images as input, accurately predicts the sites of tumours in colorectal cancer patients.





















Similar content being viewed by others
Data availability
The data were collected from publicly accessible colonoscopy datasets, namely CVC Clinic DB, Kvasir 2, and Hyper Kvasir for the project. Even though this work with internal human organs is available to the public via Internet sources, it has not been deemed ethical by official authorities. Data are publicly available from the following websites: CVC Clinic DB dataset was obtained from https://www.kaggle.com/datasets/balraj98/cvcclinicdb. Kvasir2 Dataset was obtained from https://datasets.simula.no/kvasir/. Hyper Kvasir dataset was obtained from https://datasets.simula.no/hyper-kvasir/. In this research paper, the combining of three datasets is presented as a new dataset known as a mixed dataset.
References
Murphy N, Moreno V, Hughes DJ, Vodicka L, Vodicka P, Aglago EK, Gunter MJ, Jenab M (2019) Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol Aspec Med. 1(69):2–9
Americal cancer Society, ACS. [Online]. [Accessed 15 November 2022]
Colorectal Cancer Facts & Figures 2020–2022, (2022) American Cancer Society
World Health Organization (WHO, 2018. [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/cancer. [Accessed 1 December 2022]
American Cancer Society, 2021. [Online]. Available: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html. [Accessed 2 December 2022]
Cancer Facts & Figures (2021) American cancer society
Colorectal Cancer: Risk Factors and Prevention, ASCO, May 2022. [Online]. Available: https://www.cancer.net/cancer-types/colorectal-cancer/risk-factors-and-prevention. [Accessed 20 November 2022]
Li B, Meng MQ (2012) Tumor recognition in wireless capsule endoscopy images using textural features and SVM-based feature selection. IEEE Trans Inform Technol Biomed. 16(3):323–329
Hissong E, Pittman ME (2020) Colorectal carcinoma screening: established methods and emerging technology. Crit Rev Clin Lab Sci 57(1):22–36
Issa IA, Noureddine M (2017) Colorectal cancer screening: an updated review of the available options. World J Gastroenterol 23(28):5086
Levin B, Brooks D, Smith RA, Stone A (2003) Emerging technologies in screening for colorectal cancer: CT Colonography, immunochemical fecal occult blood tests, and stool screening using molecular markers. CA A Cancer J Clin. 53(1):44–55
Taha B, Werghi N, Dias J (2017) Automatic polyp detection in endoscopy videos: A survey, In: Proceedings of the lASTED international conference biomedical engineering (BioMed 2017), Innsbruck, Aus t r i a
Borgli H, Thambawita V, Smedsrud PH, Hicks S, Jha D, Eskeland SL, Randel KR, Pogorelov K, Lux M, Nguyen DT, Johansen D (2020) HyperKvasir, a comprehensive multi-class image and video dataset for gastrointestinal endoscopy. Sci Data. 7(1):283
Tang H, Hu Z (2020) Research on medical image classification based on machine learning. IEEE Access. 8:93145–93154
Kavitha MS, Gangadaran P, Jackson A, Venmathi Maran BA, Kurita T, Ahn BC (2022) Deep neural network models for colon cancer screening. Cancers 14(15):3707
Banik D, Roy K, Bhattacharjee D, Nasipuri M, Krejcar O (2020) Polyp-net: a multimodel fusion network for polyp segmentation. IEEE Trans Instrum Measur. 10(70):1–2
Cao Z, Pan X, Yu H, Hua S, Wang D, Chen DZ, Zhou M, Wu5 J, (2022) A deep learning approach for detecting colorectal cancer via Raman spectra, BMEF
Siva Naga Raju M, Rao BS, (2022) Colorectal cancer disease classification and segmentation using a novel deep learning approach, Int J Intell Eng Syst, 4, 29
Ozturk S, ozakaya U (2021) Residual LSTM layered CNN for classification of gastrointestinal tract diseases. J Biomed Inform 113:103638
Wang Y, Feng Z, Song L, Liu X, Liu S (2021) Multiclassification of endoscopic colonoscopy images based on deep transfer learning. Comput Math Methods Med 2021:3
Souaidi M, El Ansari M (2022) A new automated polyp detection network MP-FSSD in WCE and colonoscopy images based fusion single shot multibox detector and transfer learning. IEEE Access. 10:47124–47140
Guo Q, Fang X, Wang L, Zhang E (2022) Polyp segmentation of colonoscopy images by exploring the uncertain areas. IEEE Access. 10:52971–52981
Fenlon HM, Nunes DP, Schroy PC, Barish MA, Clarke PD, Ferrucci JT (1999) A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. New Engl J Med. 341(20):1496–1503
Dominitz JA, Robertson DJ (2022) Understanding the results of a randomized trial of screening colonoscopy. N Engl J Med 387(17):1609–1611
Milletari F, Frei J, Aboulatta M, Vivar G, Ahmadi SA (2018) Cloud deployment of high-resolution medical image analysis with TOMAAT. IEEE J Biomed Health Inform 23(3):969–977
Prashanth B, Mendu M, Thallapalli R, (2021) Cloud based machine learning with advanced predictive analytics using google colaboratory, Materials Today: Proceedings
https://www.kaggle.com/balraj98/cvcclinicdb (2015) [Online]. [Accessed 25 May 2021]
https://datasets.simula.no/kvasir/, (2016) [Online]. [Accessed 3 July 2021]
https://datasets.simula.no/hyper-kvasir/, (2020) [Online]. [Accessed 3 July 2021]
Oza P, Sharma P, Patel S, Adedoyin F, Bruno A (2022) Image augmentation techniques for mammogram analysis. J Imag 8(5):141
Basher A, Kim BC, Lee KH, Jung HY (2020) Automatic localization and discrete volume measurements of hippocampi from MRI data using a convolutional neural network. IEEE Access. 8:91725–91739
Anaraki AK, Ayati M, Kazemi F (2019) Magnetic resonance imaging-based brain tumor grades classification and grading via convolutional neural networks and genetic algorithms. Biocybern Biomed Eng. 39(1):63–74
Alaskar H, Hussain A, Al-Aseem N, Liatsis P, Al-Jumeily D (2019) Application of convolutional neural networks for automated ulcer detection in wireless capsule endoscopy images. Sensors 19(6):1265
Qadir HA, Solhusvik J, Bergsland J, Aabakken L, Balasingham I (2019) A framework with a fully convolutional neural network for semi-automatic colon polyp annotation. IEEE Access. 7:169537–169547
He K, Zhang X, Ren S, Sun J (2015) Deep residual learning for image recognition, In computer vision and pattern recognition (cs.CV)
Huang G, Liu Z, Van der Maaten L (2018) Densely connected convolutional networks, computer vision and pattern recognition (cs.CV)
Ghatwary N, Ye X, Zolgharni M (2019) Esophageal abnormality detection using DenseNet based faster R-CNN with Gabor features. IEEE Access. 7:84374–84385
Simonyan K, Zisserman A (2015) Very deep convolutional networks for large-scale image recognition, Comput Vis Pattern Recognit (cs.CV),arXiv:1409.1556v6
Yang T, Liang N, Li J, Yang Y, Li Y, Huang Q, Li R, He X, Zhang H (2019) Intelligent imaging technology in diagnosis of colorectal cancer using deep learning. IEEE Access. 7:178839–178847
Li Q, Yang G, Chen Z, Bin H, Chen L, Xu D, Zhou X, Zhong S, Zhang H, Wang T (2018) Colorectal polyp segmentation using a fully convolutional neural network, In: 10th International Congress on Image and signal Processing , Biomedical Engineering and informatics (CISP-BMWI2017), Shanghai, China, 14–16
Zoph B, Vasudevan V, Shlens J, Le QV (2018) Learning transferable architectures for scalable image recognition, In: Computer vision and pattern recognition (cs.CV); Machine Learning (cs.LG); Machine Learning (stat.ML),arXiv:1707.07012v4
Agrawal T, Gupta R, Sahu S, Espy-Wilson C, (2017) SCL-UMD at the medico task-mediaeval 2017: Transfer learning based classification of medical images, In: Medico challenge, MediaEval, Dublin, Ireland
Öztürk Ş, Özkaya U (2020) Gastrointestinal tract classification using improved LSTM based CNN. Multimed Tools Appl. 79(39–40):28825–28840
Wu H, Huang Q, Wang D, Gao L (2018) A CNN-SVM combined model for pattern recognition of knee motion using mechanomyography signals. J Electromyogr Kinesiol 42:136–142
Wu W, Li D, Du J, Gao X, Gu W, Zhao F, Feng X, Yan H (2020) An intelligent diagnosis method of brain MRI tumor segmentation using deep convolutional neural network and SVM Algorithm, Computational and mathematical methods in medicine
Talo M, Baloglu UB, Yıldırım Ö, Acharya UR (2019) Application of deep transfer learning for automated brain abnormality classification using MR images. Cognit Syst Res. 54:176–188
Sampaio WB, Diniz EM, Silva AC, De Paiva AC, Gattass M (2011) Detection of masses in mammogram images using CNN, geostatistic functions and SVM. Comput Biol Med 41(8):653–664
Okamoto T, Koide T, Yoshida Hiroshi Mieno S, Toishi Takayuki H, Sugawara Masayuki T, Odagawa Nobuo Tamba M, Tamaki T, Raytchev B, Kaneda K, Tanaka S (2019) Implementation of computer-aided diagnosis system on custamizable DSP core for colorectal endoscopic images with CNN features and SVM, In: Proceedings TENCON 2018–2018 IEEE Region 10 Conference, Jeju, Korea
Thai LH, Hai TS, Thuy NT (2012) Image classification using support vector machine and artificial neural network. Int J Inform Technol Comput Sci. 4(5):32–38
Kim W, Kanezaki A, Tanaka M (2020) Unsupervised learning of image segmentation based on differentiable feature clustering. IEEE Trans Image Process 29:8055–8068
Moriya T, Roth HR, Nakamura S, Oda H, Nagara K, Oda M, Mori K (2018) Unsupervised pathology image segmentation using representation learning with spherical k-means, Medical Imaging, Digital Pathology. 10581
Liu L, Kuang L, Ji Y (2020) Multimodal MRI brain tumor image segmentation using sparse subspace clustering algorithm, Computational and Mathematical Methods in Medicine, vol 2020, p 13
Dhanachandra N, Manglem K, Chanu YJ (2015) Image segmentation using K-means clustering algorithm and subtractive clustering algorithm. Proced Comput Sci. 54:764–771
Liew WS, Tang TB, Lu CK, (2022) Computer-aided diagnostic tool for classification of colonic polyp assessment. In: International conference on artificial intelligence for smart community
Sharma P, Balabantaray BK, Bora K, Mallik S, Kasugai K, Zhao Z (2022) An ensemble-based deep convolutional neural network for computer-aided polyps identification from colonoscopy. Front Genet 13:844391
Nisha JS, Gopi VP, Palanisamy P (2022) Automated colorectal polyp detection based on image enhancement and dual-path CNN architecture. Biomed Signal Process Control 73:103465
Attallah O, Sharkas M (2021) GASTRO-CADx: a three stages framework for diagnosing gastrointestinal diseases. Peer J Comput Sci. 10(7):e423
Acknowledgements
The authors of this article are grateful to Chennai's SRM Institute of Science and Technology. Our work has been delegated to artificial intelligence, machine learning, and deep learning laboratories with advanced GPU systems. This initiative receives no funding from outside sources.
Funding
There is no external funding for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest between authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raju, A.S.N., Jayavel, K. & Rajalakshmi, T. An advanced diagnostic ColoRectalCADx utilises CNN and unsupervised visual explanations to discover malignancies. Neural Comput & Applic 35, 20631–20662 (2023). https://doi.org/10.1007/s00521-023-08859-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00521-023-08859-5