Abstract
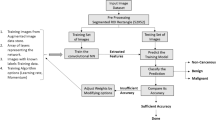
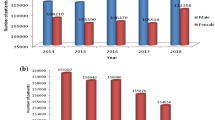
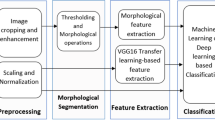
Lung cancer accounts for more than 1.5 million deaths worldwide, and it corresponded to 26% of all deaths due to cancer in 2017. However, lung computer-aided diagnosis systems developed to identify lung cancer at early stages are increasing survival rates. This study explores the performance of deep transfer learning from non-medical images on lung nodule malignancy classification tasks in order to improve such systems. Initially, the 1018 chest computed tomography (CT) examinations and medical annotations from the LIDC/IDRI were processed. Then, several convolutional neural networks (VGG16, VGG19, MobileNet, Xception, InceptionV3, ResNet50, InceptionResNetV2, DenseNet169, DenseNet201, NASNetMobile and NASNetLarge) were built, trained on the ImageNet dataset, converted into feature extractors and applied on the LIDC/IDRI nodule images. Following this, each set of deep features was submitted to 10-fold cross-validations with naive Bayes, multilayer perceptron, support vector machine (SVM), K-nearest neighbors KNN and random forest classifiers. Finally, the evaluation metrics accuracy (ACC), area under the curve (AUC), true positive rate (TPR), precision (PPV) and F1-score of each cross-validation average result were computed and compared. The results showed that the deep feature extractor based on the ResNet50 and the SVM RBF classifier, achieved an AUC metric of 93.1% (the highest value not only among the evaluated combinations, but also among the related works in the literature evaluated), a TPR of 85.38%, an ACC of 88.41%, a PPV of 73.48% and an F1-score of 78.83%. Based on these results, deep transfer learning proves to be a relevant strategy to extract representative features from lung nodule CT images.




















Similar content being viewed by others
References
Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006
de Albuquerque VHC, Damaševičius R, Garcia NM, Pinheiro PR, Filho PPR (2017) Brain computer interface systems for neurorobotics: methods and applications. BioMed Res Int 2017:1–2. https://doi.org/10.1155/2017/2505493
Armato SG, McLennan G, Bidaut L, McNitt-Gray MF, Meyer CR, Reeves AP, Zhao B, Aberle DR, Henschke CI, Hoffman EA, Kazerooni EA, MacMahon H, van Beek EJR, Yankelevitz D, Biancardi AM, Bland PH, Brown MS, Engelmann RM, Laderach GE, Max D, Pais RC, Qing DPY, Roberts RY, Smith AR, Starkey A, Batra P, Caligiuri P, Farooqi A, Gladish GW, Jude CM, Munden RF, Petkovska I, Quint LE, Schwartz LH, Sundaram B, Dodd LE, Fenimore C, Gur D, Petrick N, Freymann J, Kirby J, Hughes B, Casteele AV, Gupte S, Sallam M, Heath MD, Kuhn MH, Dharaiya E, Burns R, Fryd DS, Salganicoff M, Anand V, Shreter U, Vastagh S, Croft BY, Clarke LP (2011) The lung image database consortium (LIDC) and image database resource initiative (IDRI): a completed reference database of lung nodules on CT scans. Med Phys 38(2):915–931. https://doi.org/10.1118/1.3528204
Chollet F (2017) Xception: Deep learning with depthwise separable convolutions. In: 2017 IEEE Conference on computer vision and pattern recognition (CVPR). IEEE. https://doi.org/10.1109/cvpr.2017.195
Christodoulidis S, Anthimopoulos M, Ebner L, Christe A, Mougiakakou S (2017) Multisource transfer learning with convolutional neural networks for lung pattern analysis. IEEE J Biomed Health Inform 21(1):76–84
Cover T, Hart P (1967) Nearest neighbor pattern classification. IEEE Trans Inf Theory 13(1):21–27
Deng J, Dong W, Socher R, Li LJ, Li K, Fei-Fei L (2009) Imagenet: A large-scale hierarchical image database. In: IEEE conference on computer vision and pattern recognition, 2009. CVPR 2009. , pp. 248–255. IEEE
Dhara AK, Mukhopadhyay S, Dutta A, Garg M, Khandelwal N (2016) A combination of shape and texture features for classification of pulmonary nodules in lung ct images. J Digit Imaging 29(4):466–475
Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, Thrun S (2017) Dermatologist-level classification of skin cancer with deep neural networks. Nature 542(7639):115–118. https://doi.org/10.1038/nature21056
Filho PPR, Cortez PC, Holanda MA (2011) Modelo de contorno ativo crisp: nova técnica de segmentação dos pulmões em imagens de TC. Rev Bras Eng Bioméd 27(4):259–272. https://doi.org/10.4322/rbeb.2011.021
Filho PPR, Cortez PC, da Silva Barros AC, Albuquerque VHC, Tavares JMRS (2017) Novel and powerful 3d adaptive crisp active contour method applied in the segmentation of CT lung images. Med Image Anal 35:503–516. https://doi.org/10.1016/j.media.2016.09.002
Filho PPR, Sarmento RM, Holanda GB, de Alencar Lima D (2017) New approach to detect and classify stroke in skull CT images via analysis of brain tissue densities. Comput Methods Programs Biomed 148:27–43. https://doi.org/10.1016/j.cmpb.2017.06.011
Gupta D, Julka A, Jain S, Aggarwal T, Khanna A, Arunkumar N, de Albuquerque VHC (2018) Optimized cuttlefish algorithm for diagnosis of parkinson’s disease. Cogn Syst Res 52:36–48. https://doi.org/10.1016/j.cogsys.2018.06.006
Han F, Wang H, Zhang G, Han H, Song B, Li L, Moore W, Lu H, Zhao H, Liang Z (2015) Texture feature analysis for computer-aided diagnosis on pulmonary nodules. J Digit Imaging 28(1):99–115
Haykin S (2008) Neural networks and learning machines. Prentice Hall, McMaster University, Upper Saddle River, Hamilton
Howard AG, Zhu M, Chen B, Kalenichenko D, Wang W, Weyand T, Andreetto M, Adam H (2017) Mobilenets: efficient convolutional neural networks for mobile vision applications. CoRR arXiv:1704.04861
Hsu CW, Chang CC, Lin CJ et al (2003) A practical guide to support vector classification
Huang G, Liu Z, van der Maaten L, Weinberger KQ (2017) Densely connected convolutional networks. In: 2017 IEEE conference on computer vision and pattern recognition (CVPR). IEEE. https://doi.org/10.1109/cvpr.2017.243
Hussein S, Cao K, Song Q, Bagci, (2017) Risk stratification of lung nodules using 3d cnn-based multi-task learning. In: International conference on information processing in medical imaging, pp. 249–260. Springer
Huynh BQ, Li H, Giger ML (2016) Digital mammographic tumor classification using transfer learning from deep convolutional neural networks. J Med Imaging 3(3):034,501. https://doi.org/10.1117/1.jmi.3.3.034501
Hwang S, Kim HE, Jeong J, Kim HJ (2016) A novel approach for tuberculosis screening based on deep convolutional neural networks. In: Tourassi GD, Armato SG (eds) Medical imaging 2016: computer-aided diagnosis. SPIE, Bellingham. https://doi.org/10.1117/12.2216198
Kang G, Liu K, Hou B, Zhang N (2017) 3d multi-view convolutional neural networks for lung nodule classification. PloS ONE 12(11):e0188,290
Karpathy A, Toderici G, Shetty S, Leung T, Sukthankar R, Fei-Fei L (2014) Large-scale video classification with convolutional neural networks. In: Proceedings of the IEEE conference on computer vision and pattern recognition, pp. 1725–1732 (2014)
Kingma DP, Ba J (2014) Adam: a method for stochastic optimization. CoRR arXiv:1412.6980
Krizhevsky A, Sutskever I, Hinton GE (2012) Imagenet classification with deep convolutional neural networks. In: Advances in neural information processing systems, pp. 1097–1105
Längkvist M, Karlsson L, Loutfi A (2014) A review of unsupervised feature learning and deep learning for time-series modeling. Pattern Recognit Lett 42:11–24. https://doi.org/10.1016/j.patrec.2014.01.008
LeCun Y, Boser BE, Denker JS, Henderson D, Howard RE, Hubbard WE, Jackel LD (1990) Handwritten digit recognition with a back-propagation network. In: Advances in neural information processing systems, pp 396–404
LeCun Y, Bottou L, Bengio Y, Haffner P (1998) Gradient-based learning applied to document recognition. Proc IEEE 86(11):2278–2324
Liaw A, Wiener M et al (2002) Classification and regression by randomforest. R news 2(3):18–22
Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, van der Laak JA, van Ginneken B, Sánchez CI (2017) A survey on deep learning in medical image analysis. Med Image Anal 42:60–88. https://doi.org/10.1016/j.media.2017.07.005
Ma J, Wang Q, Ren Y, Hu H, Zhao J (2016) Automatic lung nodule classification with radiomics approach. In: SPIE medical imaging, pp 978,906–978,906. International Society for Optics and Photonics
Marcus PM, Doria-Rose VP, Gareen IF, Brewer B, Clingan K, Keating K, Rosenbaum J, Rozjabek HM, Rathmell J, Sicks J et al (2016) Did death certificates and a death review process agree on lung cancer cause of death in the national lung screening trial? Clin Trials 13(4):434–438
Näppi JJ, Hironaka T, Regge D, Yoshida H (2016) Deep transfer learning of virtual endoluminal views for the detection of polyps in CT colonography. In: Tourassi GD, Armato SG (eds) Medical imaging 2016: computer-aided diagnosis. SPIE, Bellingham. https://doi.org/10.1117/12.2217260
Pan SJ, Yang Q (2010) A survey on transfer learning. IEEE Trans Knowl Data Eng 22(10):1345–1359. https://doi.org/10.1109/tkde.2009.191
Peng H, Long F, Ding C (2005) Feature selection based on mutual information criteria of max-dependency, max-relevance, and min-redundancy. IEEE Trans Pattern Anal Mach Intell 27(8):1226–1238. https://doi.org/10.1109/tpami.2005.159
Rodrigues MB, Nóbrega RVMD, Alves SSA, Filho PPR, Duarte JBF, Sangaiah AK, Albuquerque VHCD (2018) Health of things algorithms for malignancy level classification of lung nodules. IEEE Access 6:18592–18601. https://doi.org/10.1109/ACCESS.2018.2817614
Rodrigues MB, Nobrega RVMD, Alves SSA, Filho PPR, Duarte JBF, Sangaiah AK, Albuquerque VHCD (2018) Health of things algorithms for malignancy level classification of lung nodules. IEEE Access 6:18592–18601. https://doi.org/10.1109/access.2018.2817614
Sergeeva M, Ryabchikov I, Glaznev M, Gusarova N (2016) Classification of pulmonary nodules on computed tomography scans. evaluation of the effectiveness of application of textural features extracted using wavelet transform of image. In: 2016 18th conference of open innovations association and seminar on information security and protection of information technology (FRUCT-ISPIT). IEEE. https://doi.org/10.1109/fruct-ispit.2016.7561541
Shankar K, Lakshmanaprabu SK, Gupta D, Maseleno A, de Albuquerque VHC (2018) Optimal feature-based multi-kernel SVM approach for thyroid disease classification. J Supercomput. https://doi.org/10.1007/s11227-018-2469-4
Shen W, Zhou M, Yang F, Yang C, Tian J (2015) Multi-scale convolutional neural networks for lung nodule classification. In: International conference on information processing in medical imaging, pp. 588–599. Springer
Shen W, Zhou M, Yang F, Yu D, Dong D, Yang C, Zang Y, Tian J (2017) Multi-crop convolutional neural networks for lung nodule malignancy suspiciousness classification. Pattern Recognit 61:663–673
Shie CK, Chuang CH, Chou CN, Wu MH, Chang EY (2015) Transfer representation learning for medical image analysis. In: 2015 37th annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC), pp. 711–714. IEEE
Shin HC, Roth HR, Gao M, Lu L, Xu Z, Nogues I, Yao J, Mollura D, Summers RM (2016) Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging 35(5):1285–1298. https://doi.org/10.1109/tmi.2016.2528162
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA A Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Simonyan K, Zisserman A (2014) Very deep convolutional networks for large-scale image recognition. CoRR arXiv:1409.1556
Szegedy C, Liu W, Jia Y, Sermanet P, Reed S, Anguelov D, Erhan D, Vanhoucke V, Rabinovich A (2015) Going deeper with convolutions. In: 2015 IEEE conference on computer vision and pattern recognition (CVPR). IEEE. https://doi.org/10.1109/cvpr.2015.7298594
Theodoridis S, Koutroumbas K (2008) Pattern recognition, 4th edn. Academic Press, Cambridge
Tiwari P, Qian J, Li Q, Wang B, Gupta D, Khanna A, Rodrigues JJ (2018) Detection of subtype blood cells using deep learning. Cognit Syst Res. https://doi.org/10.1016/j.cogsys.2018.08.022
Vapnik VN (1998) Statistical learning theory. Wiley, Hoboken
Wei G, Cao H, Ma H, Qi S, Qian W, Ma Z (2017) Content-based image retrieval for lung nodule classification using texture features and learned distance metric. J Med Syst 42(1):13. https://doi.org/10.1007/s10916-017-0874-5
Wei G, Ma H, Qian W, Han F, Jiang H, Qi S, Qiu M (2018) Lung nodule classification using local kernel regression models with out-of-sample extension. Biomed Signal Process Control 40:1–9
Wu S, Zhong S, Liu Y (2017) Deep residual learning for image steganalysis. Multimed Tools Appl 77(9):10437–10453. https://doi.org/10.1007/s11042-017-4440-4
Zhu W, Liu C, Fan W, Xie X (2017) Deeplung: 3d deep convolutional nets for automated pulmonary nodule detection and classification. arXiv preprint arXiv:1709.05538
Zoph B, Le QV (2016) Neural architecture search with reinforcement learning. CoRR arXiv:1611.01578
Acknowledgements
The authors acknowledge the financial support and encouragement from CNPq via Grant 304315/2017-6. The first author acknowledges the sponsorship from the Federal Institute of Education, Science and Technology of Ceará via grants PROINFRA/2017 and PROINFRA PPG/2017.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Nóbrega, R.V.M., Rebouças Filho, P.P., Rodrigues, M.B. et al. Lung nodule malignancy classification in chest computed tomography images using transfer learning and convolutional neural networks. Neural Comput & Applic 32, 11065–11082 (2020). https://doi.org/10.1007/s00521-018-3895-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00521-018-3895-1