Abstract
Background
Optimal use of bone-modifying agent (BMA) therapy in patients with bone metastases from breast and castrate-resistant prostate cancer (CRPC) is evolving.
Methods
Patients receiving BMA for bone metastases from breast or CRPC were surveyed. Information was collected on patient and disease characteristics, BMA treatments and perceptions regarding BMA benefits and side effects. Interest in participation in trials of de-escalated BMA therapy was also gauged.
Results
Of 220 patients contacted, 172 eligible patients responded (response rate 78%). Median age was 67 (range: 21–91); 137 (80%) had breast cancer and 35 (20%) CRPC. Symptomatic skeletal events (SSEs) occurred prior to starting BMAs in 61% (84/137) of breast and 48% (17/35) of CRPC patients. Among breast cancer patients, 47, 33 and 13% received zoledronate, pamidronate and denosumab, respectively. Eighty-five percent (30/35) of CRPC patients received denosumab. De-escalation of therapy was more common among breast cancer patients. Although most patients correctly reported the goals of BMA therapy were to “help stop fractures” (62%) and “[improve] quality of life” (63%), 46.5% felt it prolonged survival and 54% felt it reduced bone progression. Most respondents (102/129, 79%) were comfortable with de-escalating to 6-monthly treatment after 2 years of BMA therapy. Patients considered the most important endpoints of de-escalation studies to be “stability of bone metastases” (45%), “quality of life” (22%) and “SSE rates” (14%).
Conclusion
Twelve weekly BMA was more common in breast than in prostate cancer. There remain misconceptions about the benefits of BMAs, highlighting potential gaps in patient education. Patients were interested in further BMA de-escalation after 2 years of prior BMA and provided study endpoints that were most important to them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone is the most frequent site of metastasis for breast and prostate cancer [1,2,3]. Patients with bone metastasis can experience pain, reduced quality of life and increased mortality risks. In addition, bone metastases are associated with the composite endpoint, skeletal-related events (SRE), defined as pathological fractures, need for radiation and/or surgical intervention to bone, spinal cord compression or hypercalcemia [4]. Given its greater impact on health-related quality of life (HR-QoL), there has been a shift towards using symptomatic skeletal events (SSE), which still includes radiation or surgery to bone and spinal cord compression but excludes asymptomatic fractures and often excludes hypercalcemia given the difficulty in assessing “symptomatic hypercalcemia” [5, 6]. Bisphosphonates and denosumab are bone-modifying agents (BMAs) that reduce the incidence of SREs/SSEs and delay the time to their onset [7,8,9,10]. These agents have become an established standard of care in the treatment of breast and castrate-resistant prostate cancer (CRPC) patients with bone metastases [11,12,13,14,15].
While both the joint Cancer Care Ontario (CCO)-American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO) treatment guidelines recommend zoledronate every 4 or 12 weeks, pamidronate every 3–4 weeks or denosumab every 4 weeks from the time of diagnosis of bone metastases (or in the case of prostate cancer, at the development of bone metastases and castration-resistant disease), there is still considerable variability in practice [16, 17]. Indeed, the National Institute for Health and Care Excellence (NICE) guideline recommended against the use zoledronic acid or denosumab in prostate cancer patients [18]. Importantly, these guidelines are based on clinical trials of BMA therapy given for 1–2 years, meaning there is no high-quality evidence informing the use of BMAs beyond 2 years. Over time, it is possible that the risk of further SSEs is outweighed by the cumulative risk of BMA-related toxicities such as renal impairment, hypocalcemia and osteonecrosis of the jaw (ONJ). A recent systematic review of 12 studies noted that after 2 years of BMA therapy, the SSE rate fell while the risk of ONJ was reported to be as high as 7–18% [19]. Although frequent BMA therapy for prolonged duration increases the risk of ONJ, other risk factors include dento-alveolar surgery, tooth extractions and pre-existing inflammatory dental disease, highlighting the need for dental examinations and effective preventative care [20, 21]. These findings are particularly important as improvements in anticancer therapies mean that patients are surviving longer and commonly receiving BMAs for much longer than 2 years.
A decade ago, we published a survey of patients’ and physicians’ goals regarding BMA [22, 23]. Since then, several practice-changing trials have established BMA de-escalation from every 4 weeks to every 12 weeks as a standard treatment option [24,25,26,27]. As a result, it was deemed important by the authors to conduct an updated patient survey to assess the current pattern of BMA use in patients with bone metastases from breast and CRPC. Given the need for further study of the optimal frequency and duration after the first 2 years of BMA therapy, we sought to better understand patients’ experience and perceptions concerning their current BMA use, as well as their opinions and preferences regarding meaningful endpoints and interest in potential BMA de-escalation trial designs. The findings of this survey could be used to help improve shared decision-making in current practice and identify important patient outcomes to be included in the development of future clinical trials.
Materials and methods
Study population
The target population was patients who were currently receiving BMA therapy for bone metastases due to either breast or CRPC. The original study plan was to accrue 200 participants from 3 Canadian cancer centres (Ottawa, Thunder Bay and London). This sample size was chosen to ensure a broad perspective on treatment. Eligible patients were mainly approached during their routine clinic visits with an oncologist. Additionally, to maximize the breadth and number of patients recruited, a list of patients receiving BMA therapy at one of our treatment units in Ottawa was used to contact potential study participants. Patients had to be able to provide verbal consent and be willing and able to complete the survey, which was available in English only.
Study outcomes
Information sought in the survey aimed to obtain insights on real-world BMA prescribing practices and patient perceptions and experience of treatment. In addition, we wished to gauge patient interest in future BMA clinical trials and to determine which endpoints should be considered for such studies.
Survey development
This survey was developed by a multi-disciplinary team with demonstrated expertise in oncology, methodology and survey design [22, 23, 28,29,30]. The survey was pilot tested on a limited number of oncologists (MZA, MC, CC, TN) and two non-healthcare professionals (MS and LV) before launch. The first section of the survey collected patient demographics (age) and tumour characteristic (histology). History of SSEs prior to starting BMA was also collected with minor modifications: (1) bone pain and bone fracture were separate answer choices instead of “painful bone fracture” given a significant potential for recall bias regarding whether a bone fracture was painful, and (2) the “need for hospitalization to treat high levels of calcium in the blood” was used to capture significant hypercalcemia (Electronic Supplementary Material). The survey refers to the composite endpoint (i.e., bone pain, bone fracture, radiation to bone, surgery to bone, spinal cord compression or hypercalcemia requiring hospitalization) as bone metastasis-related complications instead. SSE rate was defined as the rate of bone metastasis-related complications minus bone pain. The type and schedule (e.g., every 4- or 12-weekly) of BMA administered in the beginning were also collected.
Section two determined information on BMA use and dosing intervals at the time of the survey, bone metastasis-related complications after starting BMAs and patient perceptions regarding the benefits of BMAs, their side effects and the impact of receiving BMA therapy on their quality of life. In the final section, respondents were asked about their attitudes towards de-escalating BMA therapy, willingness to participate in BMA de-escalation studies after 2 years of BMA therapy and potential clinical outcomes that would support de-escalation.
Survey implementation
Patients were approached to participate in the survey by either their medical oncologist or clinic nurse. Once permission was given, the study clinical research associate would contact the patient. Interested patients were provided with the option of completing a written or an electronic version of the survey. Written copies were sent via mail, with directions on return. Patients requesting electronic surveys were sent an email with a link to the anonymous survey on Microsoft Forms (on the secure, Ottawa Hospital SharePoint site), to complete online. Alternatively, patients could request to receive the questionnaire by email as a Word file or PDF file for completion. No reminders were sent to patients. The survey was approved by the Ontario Cancer Research Ethics Board (OCREB).
Data analysis
All the data were summarized descriptively. The survey consisted of close-ended multiple-choice questions which were analysed using a descriptive summary of findings in the form of frequencies and percentages using Microsoft Excel 2019 (Microsoft Corporation, Seattle, Washington) and SPSS for Mac (version v27; IBM Corp, Armonk, NY, USA).
Results
Patient characteristics
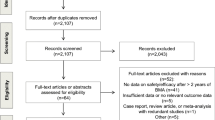
The survey was open to patients between May 13 and October 19, 2020. Unfortunately, opening of the study was delayed by the COVID-19 pandemic, which led to a slower than normal REB review and reduction in clinical research staff at all centres. As a result, two centres could not open the study and patients were therefore only accrued from the Ottawa centre. A total of 220 patients (168 breast, 52 prostate) were approached and 172 (78%) eligible candidates responded. Among the respondents, 137(80%) had breast cancer and 35 (20%) had CRPC. The median age (range) for breast cancer and CRPC respondents were 65 years (29–91) and 76 years (57–88), respectively. With respect to duration of BMA use 71/169 (42%) had been receiving BMAs for > 24 months (Table 1).
BMA treatment schedules and side effects
At the start of treatment, among breast cancer patients, 65/137 (47%) received zoledronate, 45/137 (33%) received pamidronate and 18/137 (13%) denosumab, whereas most (30/35, 85%) CRPC patients received denosumab (Table 1). At the time of initiating BMA therapy, 52/134 (39%), 71/134 (53%) and 6/134 (4%) of breast cancer patients were receiving BMA every 3–4 weeks, every 12 weeks and > 12 weekly, respectively. At the time of the survey, 18/136 (13%), 112/136 (82%) and 4/136 (3%) of breast cancer patients were receiving BMA every 3–4 weeks, every 12 weeks and > 12 weekly, respectively (Fig. 1a). For the prostate cancer subgroup, at time of initiating BMA therapy, 21/32 (66%), 6/32 (19%) and 2/32 (6%) of CRPC patients were receiving BMA every 3–4 weeks, every 12 weeks and > 12 weekly, respectively. Whereas at time of the survey, 21/34 (62%) and 12/34 (35%) of CRPC patents were receiving BMA every 3–4 weeks and every 12 weeks, respectively (Fig. 1a).
In terms of side effects, 76/137 (55%) breast cancer patients reported BMA-related side effects, including joint aches or muscle pain in 51/137 (37%), flu-like symptoms in 29/137 (21%), renal impairment in 9/137 (7%) and ONJ in 3/137 (2%). For the CRPC patients, 11/35 (31%) experienced BMA-related side effects, including joint aches or muscle pain in 7/35 (20%) patients, flu-like symptoms in 2/35 (6%) patients, renal impairment in 4/35 (11%) patients and ONJ in 1/35 (3%) patients (Table 2).
Bone metastasis-related complications prior to and after starting BMAs
Prior to starting BMA therapy, 136/172 (79%) patients reported at least one bone metastasis-related complication (108/137 [79%] of BC and 28/35 [80%] of CRPC). Among the breast cancer respondents, the most common bone metastasis-related complications were bone pain (70/137, 51%), radiation to bone (40/137, 29%) and bone fracture (25/137, 18%). Among the CRPC respondents, the most common complications were bone pain (17/35, 48%), radiation to bone (12/35, 34%) and spinal cord compression (4/35, 11%) (Table 1).
Since starting BMA therapy, 104/172 (60%) patients reported bone metastases-related complications (83/137 [61%] of breast cancer and 21/35 [60%] of CRPC patients). Among the breast cancer respondents, the most common bone complications were bone pain (54/137, 39%), radiation to bone (20/137, 14%) and spinal cord compression (11/137, 8%). Among the CRPC respondents, the most common complications were bone pain (12/35, 34%), radiation to bone (5/35, 14%) and spinal cord compression (5/35, 14%) (Table 2).
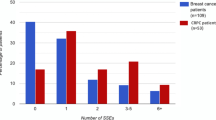
Prior to starting BMA therapy, 61/137 (44.5%) breast cancer respondents had at least one SSE, with a median of 1 SSE (36 patients had 1 SSEs, 18 patients had 2 SSEs and 7 patients had 3 SSEs), whereas after starting BMA therapy, only 33/137 (24%) breast cancer patients had at least one SSE, with a median of 1 SSE (22 patients had 1 SSE, 6 patients had 2 SSEs, 2 patients had 3 SSEs and one patient had 4 SSEs). For CRPC respondents, prior to starting BMA therapy, 15/35 (43%) had at least one SSE with a median of 1 SSE (13 patients had 1 SSE and 2 patients had 2 SSEs) whereas since starting BMA therapy, 10/35 (29%) CRPC patients had at least one SSE, with a median of 1 SSE (8 patients had 1 SSE and 2 patients had 2 SSEs).
Impact of BMA on patient lifestyle and quality of life
Most patients [119/137 (87%) breast cancer; 29/35 (83%) CRPC] received their treatment at the hospital chemotherapy unit while 4/137 (3%) of breast cancer and 4/35 (11%) of prostate cancer patients received their treatment at clinic, and 14/137 (10%) of breast cancer and 2/35 (6%) of prostate cancer patients received their treatment at home. For the breast and CRPC who received their treatment at the hospital, the total time spent in a day to receive BMA therapy was comparable between the breast and CRPC subgroups. Including commuting time, wait time, blood work and treatment time, it took less than 2 h for 76/156 (49%) patients, 2–4 h for 72/156 (46%) patients and 4–6 h for 5/156 (3%) patients. When asked if the above time included intravenous chemotherapy treatment, 73/104 (70%) of breast cancer patients were not on IV chemotherapy, 15/104 (14%) included the time of IV chemotherapy and 16/104 (16%) did not include the time of IV chemotherapy to BMA therapy duration, while 25/33 (76%) of prostate cancer patients were not on IV chemotherapy, 3/33 (9%) included the time of IV chemotherapy and 5/33 (15%) did not include the time of IV chemotherapy to BMA therapy duration.
Considering the commute, extra investigations, wait time, treatment time and potential side effects, among breast cancer respondents, 67/137 (49%) reported that BMA therapy had “no negative impact” on their lifestyle or quality of life, whereas 50/137 (36%) reported “minimal impact”, 16/137 (12%) reported “moderate impact” and 2/137 (1%) reported a “major impact” on their lifestyle or quality of life. Among prostate cancer respondents, 18/35 (51%) reported that BMA therapy had “no negative impact” on their lifestyle or quality of life, whereas 13/35 (37%) reported “minimal impact”, 3/35 (9%) reported “moderate impact” and 0/35 (0%) reported a “major impact” on their lifestyle or quality of life (Table 3).
Patient perception regarding the potential benefits of BMAs
Among breast cancer patients, the most common perceived potential benefits from BMA treatment were: "help stop fractures” (96/136, 71%), “help improve” quality of life (86/136, 63%) and “help stop cancer growing in bones” (77/136, 57%). Among prostate cancer patients, the most common perceived benefits from BMA treatment were: “help to live longer” (24/34, 71%), “help improve quality of life” (21/34, 62%) and “help stop fractures” (18/34, 53%) (Table 3).
Patient attitudes towards BMA therapy beyond 2 years
Patients were presented with a clinical scenario, whereby after completing 2 years of BMA therapy, bone metastasis-related complications became less likely while the risk of developing side effects from BMA therapy increased over time. They were then asked based on that information and their own experience, after completing 2 years of BMA therapy what frequency of BMA therapy they would be comfortable continuing. Almost all respondents (138/146, 95%) were either “somewhat comfortable” or “very comfortable”, with continuing BMA at the same frequency. Most respondents (102/129, 79%) were comfortable with de-escalating to 6 monthly treatments while a significant minority (36/121, 30%) were comfortable with discontinuing BMA (Table 4).
When asked what they felt would be the most important clinical outcome to maintain if a de-escalation study was conducted, 74/164 (45%) respondents chose “stability of bone metastases”, 36/164 (22%) chose “quality of life”, 23/164 (14%) chose “skeletal events”, 13/164 (8%) chose “physical function” and 5/164 (3%) chose “pain control” (Table 4).
When asked about their interest in participating in a randomized clinical trial evaluating the optimal frequency of BMA therapy [citing a trial that would randomize patients to either continue BMA therapy at the same frequency (every 4 or every 12 weeks) versus BMA every 24 weeks], 88/167 (53%) of breast and CRPC patients indicated a willingness to participate, 38/167 (23%) patients would not be interested and 41/167 (24%) patients were unsure (Table 4).
Discussion
Although BMAs are an established standard of care for patients with bone metastases from breast cancer and CRPC, there remains variability in practice and questions about optimal BMA duration and dosing intervals still exist [31]. The balance between the potential therapeutic benefits and side effects from long-term use of BMA therapy needs further deliberation as there is minimal prospective randomized data addressing the use of these agents beyond 2 years. This has become increasingly important as improvements in systemic anticancer therapies have extended patient survival, meaning patients are staying on BMA treatment for much longer. It also appears that, with prolonged treatment, the risk of SSEs falls [24,25,26] while the incidence of kidney impairment, hypocalcaemia and osteonecrosis of the jaw (ONJ) increases [32,33,34,35].
This survey demonstrated that Canadian breast cancer patients are more likely to receive zoledronate, whereas prostate cancer patients are more likely to receive denosumab. These differences largely reflect the funding system for these agents in Ontario where denosumab is publicly funded for CRPC but not for breast cancer. The results also showed that de-escalation of BMAs is much more common in breast cancer than CRPC patients, which is in keeping with the literature [27]. Compared with the survey from 2013, bisphosphonates remained the most common BMA used in breast cancer patients. However, more breast cancer patients are now receiving zoledronate rather than pamidronate, presumably because the cost of zoledronate now approximates that of pamidronate and requires a much shorter infusion time [22]. In the breast cohort, although 3 monthly BMA therapies were already quite prevalent in 2013, this practice has increased (current: 76% vs. 2013: 52%). Meanwhile, most prostate cancer patients continue to receive denosumab every 3–4 weeks. Concerns about reducing denosumab frequency to every 3 months include the risk of rebound in bone health deterioration and the paucity of data around denosumab de-escalation [36]. Although results of the more definitive REDUSE trial [37] are awaited, it must be noted that a recent randomized BMA de-escalation study, which included denosumab, showed non-inferiority in terms of HR-QoL physical functioning [27] and has influenced a shift towards earlier denosumab de-escalation.
Our data was consistent with the literature, confirming lower rates of SSEs after starting patients on BMA therapy compared with pre-BMA therapy. The incidence of SSEs prior to receiving BMAs among breast cancer patients in the current study was similar to that reported in a previous retrospective cohort study at our cancer centre [44.5% (61/137) and 48.4% (75/155), respectively], while the SSE rate after starting BMA was lower in the current survey [24.1% (33/137) and 47.7% (74/155), respectively] [4]. This could be attributed to advances in anti-cancer therapies that have led to more effective cancer control than before. Although the sample sizes were relatively small, especially in the CRPC cohort, and patients received a variety of BMA drugs at different schedules, the reported rates of BMA-related side effects were comparable to publicly available data [38,39,40].
Of interest, when assessing the time resources required of patients when receiving BMA therapy, most patients felt these treatments were a minimal source of burden to them. Like in our previous survey, a significant proportion of patients incorrectly perceived the use of BMA therapy would stop progression of bone metastases or improve overall survival. This reflects a potential gap in communication between physicians and patients presents an unmet need in patient education. This is important to help patients make well-informed treatment decisions.
After 2 years of prior BMA therapy, more than 75% of both breast and prostate cancer patients were at least “somewhat comfortable” with participating in further BMA de-escalation, and a significant portion of patients would even consider discontinuing BMA after 2 years. For such a de-escalation study, of the provided answer choices, the most important clinical outcome to maintain from the patient’s perspective was “stability of bone metastasis” in both breast and prostate cancer, followed by maintenance of “quality of life”. Maintenance of the rate of “skeletal events”, which is the conventional primary endpoint in large, randomized registry trials for i.v. bisphosphonates and denosumab in advanced cancer, was only chosen by 15 and 10% of breast and prostate cancer respondents, respectively. The frequent selection of “stability of bone metastasis” again implies that patients may not necessarily understand that BMAs do not have clinically significant anti-cancer activity on its own against bone metastases. Since maintenance of quality of life was voted more often than skeletal events, it would be reasonable to consider a trial using some HR-QoL measure as the primary clinical endpoint.
There are limitations to our study. Due to the COVID-19 pandemic, we were only able to open this study at one cancer centre in Ontario, thereby reflecting the practice patterns at only a single institution in one province. Nonetheless, our survey included representation from a broad age range, suggesting our sample cohort may be broadly representative. Although the survey was pre-tested on two physicians and a clinical research coordinator who did not have a healthcare background, we did not test the questionnaire on prospective patients. In terms of capturing the risk of bone-metastasis-related complications before compared to after starting BMA therapy, we did not capture the time at risk prior to starting BMA therapy (i.e. time from time of diagnosis of bone metastases to the time of starting BMA therapy). In addition, as the survey was anonymous, we could not evaluate the time at risk of SRE/SSEs prior to starting BMA therapy as this would require linking each patient to their treatment records. Similarly, information was not collected on other anticancer agents the patients had received along with their BMA therapy. Thus there could be incorrect attribution of side effects experienced by the patient.
As with all surveys, there is an inherent selection bias in those that were contacted and those that responded. Although we had a good response rate of 78%, most of the responses came from breast cancer patients, which may, in part, reflect more breast than prostate cancer patients receiving BMA overall. Of note, 126 responded by email (breast: 109 [79.6%] vs. prostate: 17 [48.6%]) and 46 on paper (breast: 28 [20.4%] vs. prostate: 18 [51.4%]), further highlighting inherent differences between the breast cancer and prostate cancer population. This issue is going to become increasingly challenging as the COVID-19 pandemic proceeds as less patients are being seen in person and more care is being delivered virtually. In order to increase the number of potential patients approached, we used pharmacy records to identify patients receiving BMAs in the local chemotherapy units. This strategy was challenging as not all patients on BMAs had bone metastases as BMAs are widely used in the adjuvant and osteoporosis settings. In addition, this data does not capture patients receiving their treatment outside of the cancer centre. Although approximately 40% of patients had received more than 2 years of BMA, for the purposes of a BMA de-escalation survey, the results of this study could be strengthened by including more patients on longer-term BMA.
Conclusion
Involvement of patients in the design of future clinical trials has become increasingly important. This survey showed there were differences in the type and frequency of BMA prescribed for patients with either breast or prostate cancer. De-escalation is more commonly used in breast cancer patients. Our results suggest that patients still have misconceptions around the reasons for receiving bone-targeted agents. Our survey suggests that patients are interested in trials of de-escalated therapy and that quality of life is a reasonable clinical endpoint to design a de-escalation study.
Data Availability
Access to data requests can be made to the Ontario Cancer Research Ethics Board (OCREB 1-866-678-6427 Ext 6649).
Code availability
N/A
References
Coleman RE (1997) Skeletal complications of malignancy. Cancer 80:1588–1594
Coleman RE, Rubens RD (1987) The clinical course of bone metastases from breast cancer. Br J Cancer 55:61–66
Hernandez RK et al (2018) Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer 18(1):44. https://doi.org/10.1186/s12885-017-3922-0
Kuchuk I et al (2013) Incidence, consequences and treatment of bone metastases in breast cancer patients- experience from a single cancer center. J Bone Oncol 2:137–144
Sartor O et al (2014) Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomised trial. Lancet Oncol 15:738–746
von Moos R et al (2016) Symptomatic skeletal events (SSEs) versus skeletal-related events (SREs) in patients with advanced cancer and bone metastases treated with denosumab or zoledronic acid. Annals Oncol 27(Supplement 6):VI507. https://doi.org/10.1093/annonc/mdw390.33
Hortobagyi GN et al (1996) Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 335:1785–91
Rosen LS et al (2001) Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: A phase III, double-blind, comparative trial. Cancer J 7:377–387
Fizazi K et al (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 27:1564–1571
Clemons M, Gelmon KA, Pritchard KI, Paterson AHG (2012) Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: The state of the art. Curr Oncol 19:259–268
Van Poznak C et al (2017) Role of bone-modifying agents in metastatic breast cancer: An American Society of Clinical oncology-cancer Care Ontario focused guideline update. J Clin Oncol 35:3978–3986
Jacobs C, Ng T, Ong M, Clemons M (2014) Long-term benefits versus side-effects from bone-targeted therapies for cancer patients: Minimizing risk while maximizing benefits. Curr Opin Support Palliat Care 8:420–428
Simos D et al (2013) Bone-Targeted Agents for the Management of Breast Cancer Patients with Bone Metastases. J Clin Med 2:67–88
Bouganim N, Dranitsaris G, Amir E, Clemons M (2011) Optimising the use of bone-targeted agents in patients with metastatic cancers: A practical guide for medical oncologists. Support Care Cancer 19:1687–1696
Bouganim N, Clemons MJ (2011) Bone-targeted agents in the treatment of bone metastases: RANK outsider or new kid on the block? Future Oncol 7:381–383
Van Poznak C, Somerfield MR, Barlow WE et al (2017) Role of bone-modifying agents in metastatic breast cancer: An American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update. J Clin Oncol 35:3978–3986
Gradishar WJ, Anderson BO, Abraham J et al (2020) Breast cancer, version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 18(4):452–478. https://doi.org/10.6004/jnccn.2020.0016
Denosumab for the prevention of skeletal-related events in adults with bone metastases from solid tumors, National Institute for Health and Care Excellence (NICE), https://www.nice.org.uk/Guidance/TA265. Published: 24 October 2012. Accessed 3 Mar 2021
Ng TL et al (2020) Long-term impact of bone-modifying agents for the treatment of bone metastases: a systematic review. Support Care Cancer. https://doi.org/10.1007/s00520-020-05556-0
Yamazaki T et al (2012) Increased incidence of osteonecrosis of the jaw after tooth extraction in patients treated with bisphosphonates: a cohort study. Int J Oral Maxillofac Surg 41:1397–1403
Vahtsevanos K et al (2009) Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol 27:5356–5362
Hutton B et al (2013) Bone-targeted agent use for bone metastases from breast cancer and prostate cancer: A patient survey. J Bone Oncol 2(3):105–9
Hutton B et al (2013) De-escalated administration of bone-targeted agents in patients with breast and prostate cancer-A survey of Canadian oncologists. J Bone Oncol 2(2):77–83
Amadori D, Aglietta M, Alessi B et al (2013) Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol 14:663–670
Himelstein AL, Foster JC, Khatcheressian JL et al (2017) Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients With Bone Metastases: A Randomized Clinical Trial. JAMA 317:48–58
Hortobagyi GN, Van Poznak C, Harker WG et al (2017) Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: The OPTIMIZE-2 Randomized Clinical Trial. JAMA Oncol 3:906–912
Clemons M et al (2020) A randomised trial of 4- versus 12-weekly administration of bone-targeted agents in patients with bone metastases from breast or castration-resistant prostate cancer. Eur J Cancer. https://doi.org/10.1016/j.ejca.2020.08.019
McGee SF et al (2019) Physician survey of timing of adjuvant endocrine therapy relative to radiotherapy in early stage breast cancer patients. Clin Breast Cancer 19:e40–e47
LeVasseur N et al (2018) Optimizing vascular access for patients receiving intravenous systemic therapy for early-stage breast cancer-a survey of oncology nurses and physicians. Curr Oncol Tor Ont 25:e298–e304
Jacobs C et al (2015) Optimisation of steroid prophylaxis schedules in breast cancer patients receiving docetaxel chemotherapy—a survey of health care providers and patients. Support Care Cancer 23:3269–3275
Fernandes R et al (2016) Future directions for bone metastasis research-Highlights from the 2015 bone and the Oncologist new updates conference (BONUS). J Bone Oncol 5(2):57–62. https://doi.org/10.1016/j.jbo.2016.02.004
Guarneri V, Donati S, Nicolini M et al (2005) Renal safety and efficacy of i.v. bisphosphonates in patients with skeletal metastases treated for up to 10 Years. Oncology 10:842–848
Henk H, Teitelbaum A, Kaura S (2012) Evaluation of the clinical benefit of long-term (beyond 2 years) treatment of skeletal-related events in advanced cancers with zoledronic acid. Curr Med Res Opin 28:1119–1127
Brufsky AM et al (2013) Long-term treatment with intravenous bisphosphonates in metastatic breast cancer: A retrospective study. Breast J 19:504–511
Stopeck AT et al (2016) Safety of long-term denosumab therapy: results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support Care Cancer 24:447–455
Lamy O, Gonzalez-Rodriguez E, Stoll D, Hans D, Aubry-Rozier B (2017) Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab 102:354–358
Gillessen S et al (2019) Incidence of hypocalcemia in patients with castration-resistant prostate cancer treated with denosumab: Data from a non-inferiority phase III trial assessing prevention of symptomatic skeletal events (SSE) with denosumab administered every four weeks (q4w) versus every 12 weeks (q12w)—SAKK 96/12 (REDUSE). J Clin Oncol 37:139–139
Bedford Laboratories. Pamidronate Disodium. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021113s017lbl.pdf. Revised: December 2014. Accessed 13 Dec 2020
Novartis Pharma Stein AG. Zometa (zoledronic acid). U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021223s028lbl.pdf. Revised: April 2014. Accessed 13 Dec 2020
Amgen Manufacturing Limited. Denosumab. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf. Revised: June 2013. Accessed 13 Dec 2020
Acknowledgements
We are grateful to patients for their participation in this survey.
Funding
The research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. This work was supported by the Rethinking Clinical Trials (REaCT) Program platform at the Ottawa Hospital which is supported by The Ottawa Hospital Foundation and its generous donors.
Author information
Authors and Affiliations
Contributions
AJM, MC, SFM, LV, GP and TN designed the study and prepared the protocol. MS and LV collected the data and coordinated the study, and AJM, TN and GP did the statistical analysis. AJM, MC, SFM, SS, MFS, AA, CC and TN enrolled patients for the survey. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. AJM, MC, TN and GP wrote the manuscript. All authors were involved in the critical review of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics committee approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of each institution Research Ethics Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Completion of the survey implied consent to participate. All data has been anonymized to protect the identities of subjects involved in the research.
Consent for publication
N/A
Conflict of interest
BH and MC reports consulting fees from Cornerstone Research, outside the submitted work. AA has participated on an advisory board for Novartis, Eli Lily, Exactis innovation and Pfizer, has received honoraria from Apobiologix and Roche and has received travel funds from Roche. CC reports travel funds from Amgen. TN reports personal fees (honoraria) from ARIAD, Takeda and Boehringer-Ingelheim, outside the submitted work. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(PDF 228 kb)
Rights and permissions
About this article
Cite this article
Alzahrani, M., Clemons, M., Sienkiewicz, M. et al. Perceptions around bone-modifying agent use in patients with bone metastases from breast and castration resistant prostate cancer: a patient survey. Support Care Cancer 29, 6903–6912 (2021). https://doi.org/10.1007/s00520-021-06238-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-021-06238-1