Abstract
Purpose
Although docetaxel plus ramucirumab has shown superior treatment efficacy over docetaxel monotherapy for patients with non-small cell lung cancer (NSCLC), the high rate of febrile neutropenia (FN) presents a clinical problem. This study aimed to validate the primary prophylactic use of pegfilgrastim with docetaxel and ramucirumab treatment in Japanese patients with NSCLC.
Methods
Patients with NSCLC with progression after at least one round of chemotherapy were enrolled and administered docetaxel (60 mg/m2) plus ramucirumab (10 mg/kg) intravenously on day 1, followed by pegylated-granulocyte colony-stimulating factor (3.6 mg) on day 2 of a 21-day treatment cycle. The primary study endpoint was the percentage of patients who developed FN. Secondary endpoints included overall survival, progression-free survival, overall response rate, and safety.
Results
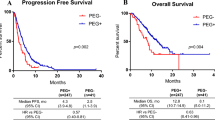
Overall, 20 patients (15 men and 5 women) were enrolled, of whom one developed FN, resulting in an overall FN rate of 5%. The response and disease control rates were 40% and 85%, respectively. The median progression-free survival was 6.6 (95% confidence interval [CI], 0.5-NR) months. The median overall survival was 18.4 (95% CI, 2.2–11.0) months. Six patients aged over 75 years were included in this study, and although most adverse events were durable, ramucirumab-associated adverse events occurred more frequently in these patients.
Conclusions
We observed a 5% FN rate using primary prophylactic pegylated-granulocyte colony-stimulating factor with docetaxel plus ramucirumab in Japanese patients with NSCLC. While most adverse events were durable, elderly patients should be closely monitored.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. https://doi.org/10.3322/caac.21442
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T, North-East Japan Study Group (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388. https://doi.org/10.1056/NEJMoa0909530
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957. https://doi.org/10.1056/NEJMoa0810699
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, Tursi J, Blackhall F, PROFILE 1014 Investigators (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371:2167–2177. https://doi.org/10.1056/NEJMoa1408440
Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SI, Perol M, Dziadziuszko R, Rosell R, Zeaiter A, Mitry E, Golding S, Balas B, Noe J, Morcos PN, Mok T, Trial Investigators ALEX (2017) Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 377:829–838. https://doi.org/10.1056/NEJMoa1704795
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. https://doi.org/10.1056/NEJMoa1504627
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, KEYNOTE-024 Investigators (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMoa1606774
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr., Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR, OAK Study Group (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265. https://doi.org/10.1016/S0140-6736(16)32517-X
Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, Leong S, O’Bryant C, Chow LQ, Serkova NJ, Meropol NJ, Lewis NL, Chiorean EG, Fox F, Youssoufian H, Rowinsky EK, Eckhardt SG (2010) Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 28:780–787. https://doi.org/10.1200/JCO.2009.23.7537
Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, Czyzewicz G, Orlov SV, Lewanski CR, Thomas M, Bidoli P, Dakhil S, Gans S, Kim JH, Grigorescu A, Karaseva N, Reck M, Cappuzzo F, Alexandris E, Sashegyi A, Yurasov S, Perol M (2014) Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 384:665–673. https://doi.org/10.1016/S0140-6736(14)60845-X
Yoh K, Hosomi Y, Kasahara K, Yamada K, Takahashi T, Yamamoto N, Nishio M, Ohe Y, Koue T, Nakamura T, Enatsu S, Lee P, Ferry D, Tamura T, Nakagawa K (2016) A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer 99:186–193. https://doi.org/10.1016/j.lungcan.2016.07.019
Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J (2010) Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 116:5555–5563. https://doi.org/10.1002/cncr.25332
Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G, Smith R, Gradishar W, Yahanda A, Vincent M, Stewart M, Glaspy J (1991) Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325:164–170. https://doi.org/10.1056/NEJM199107183250305
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL, Somlo G, Wade JL, Wozniak AJ, Armitage JO, American Society of Clinical Oncology (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33:3199–3212. https://doi.org/10.1200/JCO.2015.62.3488
Molineux G (2004) The design and development of pegfilgrastim (PEG-rmetHuG-CSF, Neulasta). Curr Pharm Des 10:1235–1244. https://doi.org/10.2174/1381612043452613
Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas-Figueroa LJ, Wiens BL, Neumann TA, Schwartzberg LS (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 23:1178–1184. https://doi.org/10.1200/JCO.2005.09.102
Kosaka Y, Rai Y, Masuda N, Takano T, Saeki T, Nakamura S, Shimazaki R, Ito Y, Tokuda Y, Tamura K (2015) Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer 23:1137–1143. https://doi.org/10.1007/s00520-014-2597-1
Kubo K, Miyazaki Y, Murayama T, Shimazaki R, Usui N, Urabe A, Hotta T, Tamura K (2016) A randomized, double-blind trial of pegfilgrastim versus filgrastim for the management of neutropenia during CHASE(R) chemotherapy for malignant lymphoma. Br J Haematol 174:563–570. https://doi.org/10.1111/bjh.14088
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Hata A, Harada D, Okuda C, Kaji R, Masuda Y, Takechi Y, Kozuki T, Nogami N, Katakami N (2018) Docetaxel plus ramucirumab with primary prophylactic pegylated-granulocyte-colony stimulating factor for pretreated non-small cell lung cancer. Oncotarget 9:27789–27796. https://doi.org/10.18632/oncotarget.25578
Mouri A, Kaira K, Shiono A, Yamaguchi O, Murayama Y, Kobayashi K, Kagamu H (2019) Clinical significance of primary prophylactic pegylated-granulocyte-colony stimulating factor after the administration of ramucirumab plus docetaxel in patients with previously treated non-small cell lung cancer. Thorac Cancer 10:1005–1008. https://doi.org/10.1111/1759-7714.13022
Ramalingam SS, Dahlberg SE, Langer CJ, Gray R, Belani CP, Brahmer JR, Sandler AB, Schiller JH, Johnson DH, Eastern Cooperative Oncology Group (2008) Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of eastern cooperative oncology group Trial 4599. J Clin Oncol 26:60–65. https://doi.org/10.1200/JCO.2007.13.1144
Wozniak AJ, Kosty MP, Jahanzeb M, Brahmer JR, Spigel DR, Leon L, Fish S, Flick ED, Hazard SJ, Lynch TJ Jr (2015) Clinical outcomes in elderly patients with advanced non-small cell lung cancer: results from ARIES, a bevacizumab observational cohort study. Clin Oncol (R Coll Radiol) 27:187–196. https://doi.org/10.1016/j.clon.2014.12.002
Laskin J, Crino L, Felip E, Franke F, Gorbunova V, Groen H, Jiang GL, Reck M, Schneider CP (2012) Safety and efficacy of first-line bevacizumab plus chemotherapy in elderly patients with advanced or recurrent nonsquamous non-small cell lung cancer: safety of avastin in lung trial (MO19390). J Thorac Oncol 7:203–211. https://doi.org/10.1097/JTO.0b013e3182370e02
Acknowledgments
The authors would like to thank Dr. Kazue Umezu from the Department of Respiratory Medicine, Gunma University Graduate School of Medicine along with Dr. Sho Osawa and Dr. Takashi Osaki for their valuable assistance in clinical work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analyses were performed by Norimitsu Kasahara, Tomohito Kuwako, Hisao Imai, and Ichiro Naruse. The first draft of the manuscript was written by Norimitsu Kasahara and all authors contributed to editing the subsequent drafts of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research involving human participants
All procedures performed in this study involving human participants were in accordance with the institutional review board of Gunma University Hospital (protocol number 1499) and with the 1964 declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kasahara, N., Sunaga, N., Kuwako, T. et al. Administration of docetaxel plus ramucirumab with primary prophylactic pegylated-granulocyte colony-stimulating factor for pretreated non-small cell lung cancer: a phase II study. Support Care Cancer 28, 4825–4831 (2020). https://doi.org/10.1007/s00520-020-05317-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05317-z