Abstract
Purpose
Sotatercept may represent a novel approach to the treatment of chemotherapy-induced anemia (CIA). We report the results from two phase 2 randomized studies examining the use of sotatercept for the treatment of CIA in patients with metastatic cancer.
Methods
In study A011-08, patients with metastatic breast cancer were randomized to 2:2:2:1 to receive sotatercept 0.1, 0.3, or 0.5 mg/kg, or placebo, respectively, every 28 days. In study ACE-011-NSCL-001, patients with solid tumors treated with platinum-based chemotherapy received sotatercept 15 or 30 mg every 42 days. The primary endpoint for both studies was hematopoietic response, defined as a hemoglobin (Hb) increase of ≥1 g/dL from baseline.
Results
Both studies were terminated early due to slow patient accrual. Among patients treated with sotatercept in the A011-08 and ACE-011-NSCL-001 studies, more patients achieved a mean Hb increase of ≥1 g/dL in the combined sotatercept 0.3 mg/kg and 15 mg (66.7 %) group and sotatercept 0.5 mg/kg and 30 mg (38.9 %) group versus the sotatercept 0.1 mg/kg (0 %) group. No patients achieved a mean Hb increase of ≥1 g/dL in the placebo group. The incidence of treatment-related adverse events (AEs) was low in both studies, and treatment discontinuations due to AEs were uncommon.
Conclusions
Although both studies were terminated early, these results indicate that sotatercept is active and has an acceptable safety profile in the treatment of CIA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced anemia (CIA) is a common complication in patients treated with myelosuppressive chemotherapy [1, 2]. Anemia is associated with fatigue, reduced functional ability, and impaired quality of life [1, 3].
Current treatment options for CIA include red blood cell (RBC) transfusions and erythropoiesis-stimulating agents (ESAs); however, both treatment types are associated with an increased risk of thrombotic events [2]. In addition, ESAs may be associated with possible decreased survival and shortened time to tumor progression in patients with cancer, and RBC transfusions carry a risk of infection, transfusion-related reactions, and possible decreased survival [2, 4–6]. Given these safety concerns, novel treatment options for CIA that are efficacious and safe are needed.
Sotatercept (ACE-011) is a recombinant fusion protein composed of the extracellular domain of the human activin receptor type IIA (ActRIIA) and the fragment crystallizable (Fc) domain of the human immunoglobulin G1 antibody [7]. Animal models and in vitro human models using RAP-011, the murine ortholog of sotatercept, have shown that sotatercept improves RBC parameters by stimulation of late-stage erythropoiesis [7]. This mechanism of action is markedly different from that of ESAs, which act on earlier stages of erythroid development [7, 8]. Sotatercept is thought to act by trapping growth differentiation factor (GDF)-11, a ligand that binds to ActRIIA and impedes terminal erythroid maturation [7, 9]. A related ligand trap fusion protein, luspatercept (ACE-536), has also been shown to promote maturation of late-stage erythroid precursors via a similar pathway [10].
Administration of sotatercept or RAP-011 has been shown to increase hemoglobin (Hb) and hematocrit levels in animal models and in healthy postmenopausal women [7, 9, 11–14]; these findings have promoted interest in sotatercept as a potential treatment for patients with anemia and other diseases characterized by ineffective erythropoiesis. In a mouse model of CIA, RAP-011 has similar effects on Hb and hematocrit [14].
Here we report the results from two phase 2 randomized studies (A011-08 and ACE-011-NSCL-001) evaluating the effects of sotatercept for the treatment of CIA. Studies A011-08 and ACE-011-NSCL-001 were performed in patients with CIA, and both studies were of similar design.
Methods
Both studies were conducted according to good clinical practice and the ethical principles outlined in the 1964 Declaration of Helsinki. All patients in both studies provided written informed consent prior to their inclusion in the studies.
Study designs
A011-08 was a phase 2, double-blind, randomized, placebo-controlled study, which assessed the efficacy, safety, and tolerability of sotatercept for the treatment of CIA in patients with metastatic breast cancer. ACE-011-NSCL-001 was a phase 2a, open-label, randomized, dose-ranging study to evaluate the efficacy and safety of sotatercept for the treatment of CIA in patients with advanced or metastatic solid tumors treated with platinum-based chemotherapy. Both trials are registered at www.ClinicalTrials.gov, A011-08 as NCT00931606 and ACE-011-NSCL-001 as NCT01284348. The data cutoff dates for studies A011-08 and ACE-011-NSCL-001 were November 18, 2010 and January 29, 2013, respectively.
Adult patients with metastatic breast cancer who were undergoing treatment with a myelosuppressive chemotherapy regimen, and who were expected to continue treatment for ≥9 weeks after initiation of study treatment, were eligible for inclusion in study A011-08. Adult patients with solid tumors and advanced or metastatic disease who were undergoing non-curative treatment with platinum-based chemotherapy were eligible for inclusion in study ACE-011-NSCL-001.
Chemotherapies were administered according to the standard of care of each study site and included anthracycline, taxane, gemcitabine, vinorelbine, or capecitabine. For both studies, eligible patients were required to have a baseline Hb level between ≥6.5 and <11.0 g/dL. Eligible patients were also required to have had no ESA treatment in the previous 30 days for study A011-08 or in the previous 28 days for study ACE-011-NSCL-001. Patients were required to have received no RBC transfusion in the previous 7 days for study A011-08 or in the previous 14 days for study ACE-011-NSCL-001. Patients in both studies were required to have received ≤2 units of transfused blood in the previous 30 days prior to study start. Patients in study A011-08 who had received >5 prior chemotherapy regimens (not including the current regimen) were excluded.
In study A011-08, patients were randomized to 2:2:2:1 to 0.1, 0.3, or 0.5 mg/kg sotatercept or to placebo, respectively; treatment was administered subcutaneously every 28 days for a maximum of four doses. Planned enrollment was 30 patients to each of the three sotatercept treatment groups and 15 patients to the placebo group. In study ACE-011-NSCL-001, patients were randomized to 15 or 30 mg of sotatercept, which was administered subcutaneously every 42 days, for a maximum of four doses; the dose schedule was chosen based on the onset and duration of Hb response to sotatercept in previous studies, as well as to accommodate common dosing schedules for platinum-based chemotherapy. Planned enrollment was up to a maximum of 30 patients, with 10–15 patients in each treatment group.
Analysis of studies
For this analysis, patients who were randomized to either sotatercept 0.3 mg/kg in study A011-08 or sotatercept 15 mg in study ACE-011-NSCL-001 have been included in a combined 0.3 mg/kg and 15 mg dose group based on an approximate body weight of 50–60 kg. Patients randomized to either sotatercept 0.5 mg/kg in study A011-08 or sotatercept 30 mg in study ACE-011-NSCL-001 have also been included in a combined sotatercept 0.5 mg/kg and 30 mg dose group. All these dose levels were considered equivalent and combined for analysis and reporting.
Dose modification
Dose modification rules were based on Hb concentrations and blood pressure readings since the last treatment (Fig. 1). Patients in study A011-08 were discontinued from treatment if they experienced drug-related toxicity of grade ≥3, with the exception of patients with grade 3 hypertension that had decreased to grade ≤1 within 7 days of receiving antihypertensive therapy. Patients in study ACE-011-NSCL-001 who experienced an Hb increase ≥3 g/dL sustained for a 28-day period received a dose reduction of two levels; of these patients, those who experienced a second Hb increase of ≥3 g/dL were discontinued from treatment. Patients in study ACE-011-NSCL-001 were also discontinued from treatment if their Hb increased to >15 g/dL, sustained for a 7-day period, whereas patients in study A011-08 with an Hb concentration above the upper limit of normal were discontinued from treatment.
Study endpoints and definition of study population
For study A011-08, the modified intent-to-treat (mITT) population and safety population included all randomized patients who received ≥1 dose of study treatment. All analyses for study ACE-011-NSCL-001 were performed on the population of treated patients, defined as randomized patients who received ≥1 dose of sotatercept.
Per protocol, the primary endpoint for study A011-08 was hematopoietic response (defined as an Hb increase from baseline of ≥1 g/dL for 28 consecutive days during the treatment period and up to 2 months after the last dose of study treatment in the absence of RBC transfusion or treatment with an ESA). In study ACE-011-NSCL-001, efficacy was also assessed by hematopoietic response, defined as an increase in Hb ≥1 g/dL from baseline that was maintained for 4 consecutive weeks in the absence of RBC transfusions and/or treatment with ESAs. For this analysis, the proportion of patients achieving a mean Hb increase from baseline of ≥1 g/dL is reported for the combined A011-08 and ACE-011-NSCL-001 study populations; mean Hb increases were calculated from the first dose received until the end of the study period.
Secondary endpoints for study A011-08 included hematopoietic response (Hb increase of ≥2 g/dL and Hb concentration ≥11 g/dL sustained for 28 consecutive days), time to achieve hematopoietic response, duration of hematopoietic response, the proportion of patients requiring RBC transfusion and/or treatment with an ESA, and safety. Exploratory endpoints for ACE-011-NSCL-001 included the evaluation of the duration of hematopoietic response, assessment of renal function biomarkers, and evaluation of quality of life and bone metabolism. Safety was evaluated in A011-08 using the safety population, whereas all other secondary endpoints for A011-08 were evaluated in the mITT population. Adverse events (AEs) were recorded according to National Cancer Institute Common Terminology Criteria for Adverse Events guidelines version 3.0 and version 4.0 for the A011-08 and ACE-011-NSCL-001 studies, respectively.
Results
Both studies were terminated early due to slower than expected patient accrual rates. Accrual rates were slower than expected due to recent substantial changes in the standard of care for patients with cancer and anemia. Therefore, all analyses presented are exploratory.
Patients
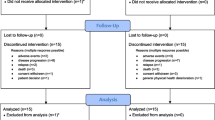
Of 55 patients enrolled in the two studies, 5 patients received placebo and 50 patients received ≥1 dose of sotatercept: 8 patients received sotatercept 0.1 mg/kg, 24 patients received either sotatercept 0.3 mg/kg or 15 mg, and 18 patients received either sotatercept 0.5 mg/kg or 30 mg. A total of 30 patients were enrolled as part of study A011-08 and 25 patients were enrolled and received ≥1 dose of study treatment in study ACE-011-NSCL-001.
Baseline characteristics are presented in Table 1. For patients treated with sotatercept, median age was 57 years (range 22–81 years), 39 patients (78.0 %) were female, and median Hb level at baseline was 9.0 g/dL (range 6.3–11.0). Prior and concomitant antineoplastic use is presented in Table 2; carboplatin was the most frequently administered concurrent chemotherapy regimen in patients treated with sotatercept.
Efficacy
Efficacy results are presented in Table 3. A mean Hb increase of ≥1 g/dL was observed for 7 of 18 patients (38.9 %) in the combined sotatercept 0.5 mg/kg and 30 mg dose groups, 16 of 24 patients (66.7 %) in the combined sotatercept 0.3 mg/kg and 15 mg dose groups, 0 of 8 patients in the sotatercept 0.1 mg/kg dose group, and 0 of 5 patients treated with placebo.
A mean increase in Hb levels ≥2 g/dL was observed in 2 of 18 patients (11.1 %) in the combined sotatercept 0.5 mg/kg and 30 mg dose group, 2 of 24 patients (8.3 %) in the combined sotatercept 0.3 mg/kg and 15 mg dose group, 0 of 8 patients in the sotatercept 0.1 mg/kg dose group, and 0 of 5 patients treated with placebo (Fig. 2).
Of the 13 non-responders who received sotatercept in study A011-08, 5 patients (38.5 %) had ≥1 dose interruption or reduction. In four of these patients (80.0 %), an initial Hb increase of ≥1 g/dL from baseline on or before day 29 had been documented; however, following interruption/reduction of the second sotatercept dose, the initial Hb increase was not sustained. Mean Hb level increase following the first dose of study treatment peaked at approximately 15 days after administration in the 0.3 and 0.5 mg/kg dose groups, before decreasing to near baseline levels by day 29.
Overall, 18 patients (36.0 %) administered sotatercept required either RBC transfusion or ESAs during the studies reported herein: 9 patients (50.0 %) in the combined sotatercept 0.5 mg/kg and 30 mg group, 8 patients (33.3 %) in the combined sotatercept 0.3 mg/kg and 15 mg group, and 1 patient (12.5 %) patient in the sotatercept 0.1 mg/kg group. Due to the small number of responses, median time to response was not evaluable.
Safety
Of the 50 patients who received sotatercept, 10 (20.0 %) received the planned four doses of study drug: 2 patients (11.1 %) in the combined 0.5 mg/kg and 30 mg dose groups, 7 patients (29.2 %) in the combined 0.3 mg/kg and 15 mg dose groups, and 1 patient (12.5 %) in the 0.1 mg/kg dose group. A further 8 patients (16.0 %) received three doses of sotatercept, 26 patients (52.0 %) received two doses, and 6 patients (12.0 %) received one dose.
AEs were reported in 44 patients (88.0 %) and 5 patients (100 %) administered sotatercept and placebo, respectively (Table 4). Grade ≥3 AEs were reported in 35 patients (70.0 %) and 4 patients (80.0 %) administered sotatercept and placebo, respectively. The most commonly reported grade ≥3 AEs occurring in more than 10 % of patients were anemia, neutropenia, and thrombocytopenia in sotatercept-treated patients and neutropenia in placebo-treated patients. One patient in the combined sotatercept 0.5 mg/kg and 30 mg dose groups reported a grade ≥3 thrombotic event (cerebrovascular accident). AEs related to study treatment were reported in 11 patients (22.0 %) treated with sotatercept.
Overall, 18 patients (36.0 %) died: 8 patients died in the combined sotatercept 0.5 mg/kg and 30 mg dose groups, 9 patients died in the combined sotatercept 0.3 mg/kg and 15 mg dose groups, and 1 patient died in the sotatercept 0.1 mg/kg dose group; no patient died in the placebo group. Cause of death was reported as disease progression for 16 patients and unknown causes for 2 patients (a 57-year-old female in the combined 0.3 mg/kg and 15 mg dose group and a 72-year-old female in the combined 0.5 mg/kg and 30 mg dose group). Four deaths occurred during the on-treatment period, all due to disease progression; the remaining 14 deaths all occurred more than 30 days after the last dose of sotatercept. No treatment-related deaths were reported, and no AEs leading to death were considered study-drug-related.
Of the 50 patients who received sotatercept, 19 (38.0 %) had ≥1 dose interruption or reduction during the study treatment period per protocol. A total of eight patients (44.4 %) in the combined sotatercept 0.5 mg/kg and 30 mg dose groups, seven patients (29.2 %) in the combined sotatercept 0.3 mg/kg and 15 mg dose groups, and four patients (50 %) in the 0.1 mg/kg dose group had ≥1 dose interruption or reduction; a further three patients (60.0 %) treated with placebo had ≥1 dose interruption or reduction. Dose delay or interruption due to AEs was experienced by three patients (16.7 %) in the combined sotatercept 0.5 mg/kg and 30 mg dose group: one patient experienced thrombocytopenia, one patient experienced hypertension, and one patient experienced pathologic fractures.
Treatment discontinuation as a result of AEs occurred in eight patients (16.0 %) treated with sotatercept: six patients in the combined 0.5 mg/kg and 30 mg dose groups (metastatic breast cancer, tumor hemorrhage, gastric ulcer perforation, pathological fracture, grade 3 muscular weakness, and grade 1 malignant mediastinal neoplasm), one patient in the combined 0.3 mg/kg and 15 mg dose groups (grade 3 acute renal failure), and one patient in the 0.1 mg/kg dose group (anemia)—none was considered to be related to study treatment. Treatment was discontinued for one patient (20.0 %) treated with placebo (fatigue).
Discussion
Sotatercept has multiple direct and indirect hematologic effects that may contribute to its ability to improve the symptoms of anemia [15–18].
In a previous study of healthy postmenopausal women, sotatercept administration was associated with marked increases in Hb, hematocrit, and RBC counts; sotatercept has been suggested as a potential treatment for patients with ineffective erythropoiesis [13]. Furthermore, preliminary data from another study have demonstrated evidence of sotatercept activity in patients with β-thalassemia, a condition in which ESAs are generally ineffective [8, 19]. These data suggest a mechanism of action for sotatercept distinct from that of ESAs and, therefore, raise the possibility of its use in other disease areas.
The two phase 2 studies described herein evaluated sotatercept as a potential treatment for CIA. The studies were of a similar design, with similar endpoints, populations, and dose-modification rules. A mean Hb increase from baseline of ≥1 g/dL was observed in 46 % of sotatercept-treated patients with CIA, whether they had metastatic breast cancer (study A011-08) or metastatic solid tumors (study ACE-011-NSCL-001). Higher response rates were generally reported in the combined sotatercept 0.3 mg/kg and 15 mg dose group and the combined sotatercept 0.5 mg/kg and 30 mg dose group versus the sotatercept 0.1 mg/kg group, suggesting a possible dose–response relationship.
In both studies, sotatercept was well tolerated in patients with CIA; safety findings were comparable to placebo and were generally consistent with the disease under study [11, 13]. The incidence of treatment-related AEs was low in both studies, and treatment discontinuations due to AEs were comparable to placebo. A single grade ≥3 thrombotic event was reported in the combined sotatercept 0.5 mg/kg and 30 mg dose group. Other treatment options for CIA, including ESAs and RBC transfusion, are associated with an increased risk of thrombotic events [2]. More deaths were reported in the sotatercept treatment groups versus the placebo group; the majority of deaths were due to disease progression. Given the small number of patients in the placebo group, mortality rates in the placebo and sotatercept treatment groups could not be meaningfully compared. The generally favorable safety profile of sotatercept highlights its potential as an alternative to ESAs in the treatment of CIA. However, due to the small number of patients enrolled and the limited follow-up data available, it was not possible to assess the effect of sotatercept on tumor progression.
There are several limitations to these studies. It is likely that the dose-modification rules used may have resulted in patients who would otherwise have achieved a hematopoietic response being categorized as non-responders; indeed, the majority of patients who had a dose interruption or reduction in study A011-08 achieved an initial Hb increase from baseline of ≥1 g/dL that was not sustained after dose modification.
Although the optimal dose and schedule of sotatercept for the treatment of CIA has not been established, it is likely that the dose scheduling employed in our studies may have affected outcomes. The two studies reported herein used dosing intervals of 28 and 42 days, respectively; the dosing schedules used were selected based on preliminary pharmacokinetics and pharmacodynamics data from healthy volunteers treated with sotatercept; mean terminal half-life after subcutaneous multiple dose administration of sotatercept was approximately 23 days, and Hb increase was sustained until ≥2 months after the last dose of sotatercept. Previous studies involving healthy volunteers have also shown sustained Hb increases associated with subcutaneous administration of sotatercept once every 28 days [13]. However, Hb increases peaked 15 days after administration of the first dose of study treatment in study A011-08, with a decrease to baseline levels by day 29, suggesting that the lack of a sustained Hb response may be due to the dosing schedule used. Most patients in study ACE-011-NSCL-001 had a hematological response, although responses did not always meet study criteria and were not sustained. These results suggest that shorter dosing intervals may be beneficial in future studies.
Based on preliminary pharmacokinetics and pharmacodynamics data from healthy volunteers, the doses administered in these studies did not exceed 0.5 mg/kg administered once every 28 days. However, subsequent ongoing studies with sotatercept have exceeded this dose: patients with anemia are currently undergoing treatment with sotatercept >1 mg/kg administered once every 3 weeks, with no dose-limiting toxicities, and the maximum tolerated dose has not yet been reached. It is possible, therefore, that the sotatercept doses used herein may be beneath an optimal threshold.
The use of concomitant chemotherapy regimens was not controlled for in either study and may have potentially affected study outcomes.
Both of the reported studies were terminated early due to slow patient accrual as a result of significant changes in the recommended treatment of CIA, as opposed to insufficient efficacy or safety concerns. Consequently, the number of patients enrolled was small and the findings should be interpreted with caution.
Despite these limitations, the available data provide insights into the potential clinical efficacy and safety of sotatercept in the treatment of CIA. The results of these studies indicate that sotatercept is active and has an acceptable safety profile when used in the treatment of CIA. Furthermore, preliminary data from phase 2 trials of sotatercept for the treatment of anemia in patients with diseases characterized by ineffective erythropoiesis, including β-thalassemia and myelodysplastic syndromes, are encouraging, further supporting the clinical potential of sotatercept [19, 20].
References
Groopman JE, Itri LM (1999) Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst 91:1616–1634
National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Cancer- and chemotherapy-related anemia. Version 2.2014. www.nccn.org. Accessed 13 Nov 2014
Cella D, Lai JS, Chang CH, Peterman A, Slavin M (2002) Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 94:528–538
Henke M, Laszig R, Rübe C, Schäfer U, Haase KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, Frommhold H (2003) Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet 362:1255–1260
Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, Makhson A, Roth A, Dodwell D, Baselga J, Biakhov M, Valuckas K, Voznyi E, Liu X, Vercammen E (2005) Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol 23:5960–5972
Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, Barnato SE, Elverman KM, Courtney DM, McKoy JM, Edwards BJ, Tigue CC, Raisch DW, Yarnold PR, Dorr DA, Kuzel TM, Tallman MS, Trifilio SM, West DP, Lai SY, Henke M (2008) Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 299:914–924
Carrancio S, Markovics J, Wong P, Leisten J, Castiglioni P, Groza MC, Raymon HK, Heise C, Daniel T, Chopra R, Sung V (2014) An activin receptor IIa ligand trap promotes erythropoiesis resulting in a rapid induction of red blood cell number and hemoglobin. Br J Haematol 165:870–882
Cappellini M-D, Porter J, Origa R, Forni GL, Laadem A, Galacteros F, Miteva D, Sung V, Chopra R, Arlet J-B, Ribeil J-A, Klesczewski K, Attie K, Garbowski M, Hermine O (2013) A phase 2a, open-label, dose-finding study to determine the safety and tolerability of sotatercept (ACE-011) in adults with beta (β)-thalassemia: interim results. Blood 122:abstract 3448
Dussiot M, Maciel TT, Fricot A, Chartier C, Negre O, Veiga J, Grapton D, Paubelle E, Payen E, Beuzard Y, Leboulch P, Ribeil JA, Arlet JB, Coté F, Courtois G, Ginzburg YZ, Daniel TO, Chopra R, Sung V, Hermine O, Moura IC (2014) An activin receptor IIA ligand trap corrects ineffective erythropoiesis in β-thalassemia. Nat Med 20:398–407
Suragani RN, Cadena SM, Cawley SM, Sako D, Mitchell D, Li R, Davies MV, Alexander MJ, Devine M, Loveday KS, Underwood KW, Grinberg AV, Quisel JD, Chopra R, Pearsall RS, Seehra J, Kumar R (2014) Transforming growth factor-β superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat Med 20:408–414
Ruckle J, Jacobs M, Kramer W, Pearsall AE, Kumar R, Underwood KW, Seehra J, Yang Y, Condon CH, Sherman ML (2009) Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res 24:744–752
Chen N, Laadem A, Sherman ML, Zhou S, Sung V, Palmisano M, Chopra R (2012) Exposures and erythropoietic responses to sotatercept (ACE-011) in healthy volunteers and cancer patients: implications for mechanism of action. Blood 120:abstract 3454
Sherman ML, Borgstein NG, Mook L, Wilson D, Yang Y, Chen N, Kumar R, Kim K, Laadem A (2013) Multiple‐dose, safety, pharmacokinetic, and pharmacodynamic study of sotatercept (ActRIIA‐IgG1), a novel erythropoietic agent, in healthy postmenopausal women. J Clin Pharmacol 53:1121–1130
Mulivor AW, Barbosa D, Kumar R, Sherman ML, Seehra J, Pearsall RS (2009) RAP-011, a soluble activin receptor type IIa murine IgG-Fc fusion protein, prevents chemotherapy induced anemia. Blood 114:abstract 161
Abdulkadyrov KM, Salogub GN, Khuazheva NK, Sherman ML, Laadem A, Barger R, Knight R, Srinivasan S, Terpos E (2014) Sotatercept in patients with osteolytic lesions of multiple myeloma. Br J Haematol 165:814–823
Raje N, Vallet S (2010) Sotatercept, a soluble activin receptor type 2A IgG-Fc fusion protein for the treatment of anemia and bone loss. Curr Opin Mol Ther 12:586–597
Iancu-Rubin C, Mosoyan G, Wang J, Kraus T, Sung V, Hoffman R (2013) Stromal cell-mediated inhibition of erythropoiesis can be attenuated by sotatercept (ACE-011), an activin receptor type II ligand trap. Exp Hematol 41:155–166
Fields SZ, Parshad S, Anne M, Raftopoulos H, Alexander MJ, Sherman ML, Laadem A, Sung V, Terpos E (2013) Activin receptor antagonists for cancer-related anemia and bone disease. Expert Opin Investig Drugs 22:87–101
Porter J, Cappellini MD, Origa R, Forni GL, Laadem A, Galacteros F, Voskaridou E, Miteva D, Sung V, Chopra R, Arlet JB, Ribeil JA, Klesczewski K, Attie K, Garbowski M, Graziadei G, Balocco M, Hermine O (2014) Interim results from a phase 2a, open-label, dose-finding study to determine the safety, efficacy, and tolerability of sotatercept (ACE-011) in adults with beta-thalassemia. Haematologica 99(Suppl 1):abstract S662
Komrokji R, Garcia-Manero G, Ades L, Laadem A, Vo B, Prebet T, Stamatoullas A, Boyd T, Delaunay J, Steensma DP, Sekeres MA, Beyne-Rauzy O, Zou J, Attie K, Sherman ML, Fenuax P, List AF (2014) An open-label, phase 2, dose-finding study of sotatercept (ACE-011) in patients with Low or Intermediate-1 (Int-1)-risk myelodysplastic syndromes (MDS) or non-proliferative chronic myelomonocytic leukemia (CMML) and anemia requiring transfusion. Blood 124:abstract3251
Acknowledgments
The authors would like to thank Kenneth Klesczewski for providing statistical input for the analyses. Celgene Corporation provided funding for this study. The authors received editorial and writing support provided by James O’Reilly, PhD, from Excerpta Medica, funded by Celgene Corporation. The authors had full access to the data and are fully responsible for content and editorial decisions for this manuscript.
Conflict of interest
Haralambos Raftopoulos and Paul J. Hesketh have received research funding from Celgene Corporation. Abderrahmane Laadem and Jun Zou are employees of and hold equity in Celgene Corporation. Jerome H Goldschmidt is a consultant/advisor for Roche, Genentech, Bristol Myers Squibb, and Celgene Corporation. Matthew L. Sherman is an employee of and holds equity in Acceleron Pharma. Marie-Puccio Pick is an employee of and holds equity in Celgene Corporation and holds equity in Novartis. Jeffrey Crawford is a consultant/advisor for Celgene Corporation. Nashat Gabrail, Cynthia Osborne, Muhammad Ali, Ding Wang, and John Glaspy have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Raftopoulos, H., Laadem, A., Hesketh, P.J. et al. Sotatercept (ACE-011) for the treatment of chemotherapy-induced anemia in patients with metastatic breast cancer or advanced or metastatic solid tumors treated with platinum-based chemotherapeutic regimens: results from two phase 2 studies. Support Care Cancer 24, 1517–1525 (2016). https://doi.org/10.1007/s00520-015-2929-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2929-9