Abstract
Purpose
Lipegfilgrastim is a once-per-cycle glycoPEGylated granulocyte colony-stimulating factor (G-CSF). Noninferiority of lipegfilgrastim versus pegfilgrastim was demonstrated in a phase III trial in chemotherapy (CTx)-naïve breast cancer patients. Secondary outcomes relating to treatment burden are reported here.
Methods
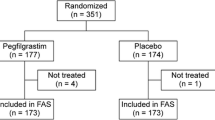
Patients with high-risk stage II, III, or IV breast cancer were randomized to receive lipegfilgrastim 6 mg (n = 101) or pegfilgrastim 6 mg (n = 101) subcutaneously on day 2 of each CTx cycle. Doxorubicin 60 mg/m2 plus docetaxel 75 mg/m2 commenced on day 1, for up to four cycles. Secondary end points included days in the hospital or intensive care unit (ICU), use of intravenous antibiotics for febrile neutropenia (FN) or related infections, and measures of CTx delivery (dose delays, reductions, and omissions).
Results
One lipegfilgrastim recipient and two pegfilgrastim recipients were hospitalized in cycle 1 because of FN or associated infection. The lipegfilgrastim-treated patient spent 1 day in the ICU for FN, and the two pegfilgrastim-treated patients were hospitalized for FN for 5 and 6 days, respectively. All hospitalized patients received antibiotics. An additional pegfilgrastim-treated patient received antibiotics but was not hospitalized. Most patients received CTx as scheduled; over 98 % received their planned doxorubicin and docetaxel doses in all cycles. In the lipegfilgrastim group, no patients had a CTx dose reduced or omitted; eight patients in the pegfilgrastim group had a CTx dose reduced or omitted during cycles 2–4.
Conclusions
The burden of treatment associated with myelosuppressive CTx was similar in breast cancer patients treated with lipegfilgrastim or pegfilgrastim.
Similar content being viewed by others
References
Crawford J, Dale DC, Lyman GH (2004) Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 100:228–237. doi:10.1002/cncr.11882
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266. doi:10.1002/cncr.21847
Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL (2011) Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer 11:404. doi:10.1186/1471-2407-11-404
Crawford J, Caserta C, Roila F (2010) Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol 21(suppl 5):v248–v251. doi:10.1093/annonc/mdq195
Aapro MS, Bohlius J, Cameron DA et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32. doi:10.1016/j.ejca.2010.10.013
NCCN Clinical Practice Guidelines in Oncology (2014) Myeloid Growth Factors v.2.2014. National Comprehensive Cancer Network. www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 1 May 2015
Smith TJ, Khatcheressian J, Lyman GH et al (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:3187–3205. doi:10.1200/JCO.2006.06.4451
Neupogen [package insert] (2013) Amgen Inc., Thousand Oaks, CA
Yang BB, Kido A (2011) Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet 50:295–306. doi:10.2165/11586040-000000000-00000
Neulasta [package insert] (2014) Amgen Inc., Thousand Oaks, CA
Lonquex: Summary of Product Characteristics. (2013) European Medicines Agency, London, UK
Bondarenko I, Gladkov OA, Elsaesser R, Buchner A, Bias P (2013) Efficacy and safety of lipegfilgrastim versus pegfilgrastim: a randomized, multicenter, active-control phase 3 trial in patients with breast cancer receiving doxorubicin/docetaxel chemotherapy. BMC Cancer 13:386–398. doi:10.1186/1471-2407-13-386
Buchner A, Elsasser R, Bias P (2014) A randomized, double-blind, active control, multicenter, dose-finding study of lipegfilgrastim (XM22) in breast cancer patients receiving myelosuppressive therapy. Breast Cancer Res Treat 148:107–116. doi:10.1007/s10549-014-3120-6
Montella L, Addeo R, Guarrasi R et al (2010) Once-per-cycle pegfilgrastim in breast cancer patients treated with docetaxel/epidoxorubicin/cyclophosphamide. Eur J Cancer Care (Engl) 19:200–204. doi:10.1111/j.1365-2354.2008.01004.x
Ngamphaiboon N, O'Connor TL, Advani PP, Levine EG, Kossoff EB (2012) Febrile neutropenia in adjuvant docetaxel and cyclophosphamide (TC) with prophylactic pegfilgrastim in breast cancer patients: a retrospective analysis. Med Oncol 29:1495–1501. doi:10.1007/s12032-011-0035-5
Ozer H, Mirtsching B, Rader M et al (2007) Neutropenic events in community practices reduced by first and subsequent cycle pegfilgrastim use. Oncologist 12:484–494. doi:10.1634/theoncologist.12-4-484
Siena S, Piccart MJ, Holmes FA, Glaspy J, Hackett J, Renwick JJ (2003) A combined analysis of two pivotal randomized trials of a single dose of pegfilgrastim per chemotherapy cycle and daily filgrastim in patients with stage II–IV breast cancer. Oncol Rep 10:715–724
von Minckwitz G, Kummel S, du Bois A et al (2008) Pegfilgrastim +/− ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol 19:292–298. doi:10.1093/annonc/mdm438
Aapro M, Schwenkglenks M, Lyman GH et al (2010) Pegfilgrastim primary prophylaxis vs. current practice neutropenia management in elderly breast cancer patients receiving chemotherapy. Crit Rev Oncol Hematol 74:203–210. doi:10.1016/j.critrevonc.2009.06.004
Holmes FA, O'Shaughnessy JA, Vukelja S et al (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 20:727–731
von Minckwitz G, Schwenkglenks M, Skacel T et al (2009) Febrile neutropenia and related complications in breast cancer patients receiving pegfilgrastim primary prophylaxis versus current practice neutropaenia management: results from an integrated analysis. Eur J Cancer 45:608–617. doi:10.1016/j.ejca.2008.11.021
Naeim A, Henk HJ, Becker L et al (2013) Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer 13:11. doi:10.1186/1471-2407-13-11
Weycker D, Malin J, Kim J et al (2009) Risk of hospitalization for neutropenic complications of chemotherapy in patients with primary solid tumors receiving pegfilgrastim or filgrastim prophylaxis: a retrospective cohort study. Clin Ther 31:1069–1081. doi:10.1016/j.clinthera.2009.05.019
Klastersky J, Awada A (2011) Prevention of febrile neutropenia in chemotherapy-treated cancer patients: Pegylated versus standard myeloid colony stimulating factors. Do we have a choice? Crit Rev Oncol Hematol 78:17–23. doi:10.1016/j.critrevonc.2010.02.005
Vogel CL, Wojtukiewicz MZ, Carroll RR et al (2005) First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 23:1178–1184. doi:10.1200/JCO.2005.09.102
Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT (2005) Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 103:1916–1924. doi:10.1002/cncr.20983
Johnson P, Bancroft T, Barron R et al (2014) Discrete choice experiment to estimate breast cancer patients' preferences and willingness to pay for prophylactic granulocyte colony-stimulating factors. Value Health 17:380–389. doi:10.1016/j.jval.2014.01.002
Fortner BV, Tauer K, Zhu L et al (2004) Medical visits for chemotherapy and chemotherapy-induced neutropenia: a survey of the impact on patient time and activities. BMC Cancer 4:22. doi:10.1186/1471-2407-4-22
Jenkins P, Scaife J, Freeman S (2012) Validation of a predictive model that identifies patients at high risk of developing febrile neutropaenia following chemotherapy for breast cancer. Ann Oncol 23:1766–1771. doi:10.1093/annonc/mdr493
Acknowledgments
This study was sponsored by Teva Branded Pharmaceutical Products R&D, Inc. Medical writing assistance was provided by Laurie Orloski, PharmD and Lisa Feder, PhD of Peloton Advantage and was funded by Teva Branded Pharmaceutical Products R&D, Inc. Teva provided a full review of the article.
Conflict of interest
Oleg A. Gladkov declares that he has no conflict of interest. Anton Buchner is an employee of Merckle GmbH. Peter Bias, Udo Müller, and Reiner Elsässer are employees of Teva Ratiopharm.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gladkov, O.A., Buchner, A., Bias, P. et al. Chemotherapy-associated treatment burden in breast cancer patients receiving lipegfilgrastim or pegfilgrastim: secondary efficacy data from a phase III study. Support Care Cancer 24, 395–400 (2016). https://doi.org/10.1007/s00520-015-2803-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2803-9