Abstract
Purpose
Oral mucositis (OM) is a frequent side effect resulting from antineoplastic treatment and is described as an acute alteration characterized by ulcerative lesions, with the presence of a persistent chronic inflammatory infiltrate, erythema, and pain.
Aims
The purpose of the study was to evaluate the presence of the herpes simplex virus (HSV-1/2) in patients with squamous cell carcinoma of the head and neck region (SCC) and its influence on the aggravation of oral mucositis after radiotherapy or radio/chemotherapy treatment.
Methods
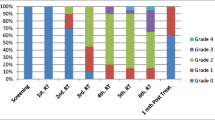
In this prospective cohort study, 91 patients were evaluated with regard to their serological status for IgG before treatment (initial time interval—TI) and for IgM before treatment (T1) and on the 30th day after the first day of radiotherapy application/radiation therapy (final time interval—TF), using immunoenzymatic assay (ELISA), and the results were correlated with the intensity of OM.
Results
The seroprevalence for IgG was 97.8 %. IgM (TI) was positive in 18.7 % and IgM (TF) in 20.9 % of patients. All the patients developed some degree of oral mucositis; however, there was statistically significant correlation between positivity for IgM and degree of severity of OM, irrespective of the type of treatment to which the patient was submitted.
Conclusion
The reactivation of HSV-1/2 was shown to be relatively infrequent and there was no correlation between presence of the virus and aggravation of oral mucositis resulting from antineoplastic treatment.
Similar content being viewed by others
References
Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45:309–316
Baskar R, Lee KM, Yeo R, Yeoh KW (2012) Cancer and radiation therapy: current advances and future directions. Int J Med Sci 9:193–199
Wong PC, Dodd MJ, Miaskowski C, Paul SM, Bank KA, Shiba GH, Facione N (2006) Mucositis pain induced by radiation therapy: prevalence, severity, and use of self-care behaviors. J Pain Symptom Manag 32:27–37
Basu T, Laskar SG, Gupta T, Budrukkar A, Murthy V, Agarwal JP (2012) Toxicity with radiotherapy for oral cancers and its management: a practical approach. J Cancer Res Ther 8(Suppl 1):S72–S84
Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4:277–284
Trotti A, Bellm LA, Epstein JB (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 66:253–262
Khan SA, Wingard JR (2001) Infection and mucosal injury in cancer treatment. J Natl Cancer Inst Monogr 29:31–36
Treister N, Sonis ST (2007) Mucositis: biology and management. Curr Opin Otolaryngol Head Neck Surg 15:123–129
Meyers JD, Flourney N, Thomas ED (1981) Infection with herpes simplex virus and cell-mediated immunity after marrow transplant. J Infect Dis 142:338–346
Saral R, Burns WH, Laskin OL, Santos GW (1981) Acyclovir prophylaxis of herpes-simplex-virus infections. N Engl J Med 305:63–67
Barrett A (1986) A long-term prospective clinical study of orofacial herpes simplex virus infection in acute leukemia. Oral Surc Oral Med Oral Pathol 61:149–152
Montgomery M, Redding S, LeMaistre C (1986) The incidence of oral herpes simplex virus infection in patients undergoing cancer chemotherapy. Oral Surg Oral Med Oral Pathol 61:238–242
Greenberg M, Cohen S, Boosz B, Friedman H (1987) Oral herpes simplex virus infections in patients with leukemia. J Am Dent Assoc 114:483–486
Epstein JB, Sherlock C, Page JL, Spinelli J, Phillips G (1990) Clinical study of herpes simplex virus infection in leukemia. Oral Surg Oral Med Oral Pathol 70:38–43
Schubert MM, Peterson DE, Flournoy N, Meyers JD, Truelove EL (1990) Oral and pharyngeal herpes simplex virus infection after allogeneic bone marrow transplantation: analysis of factors associated with infection. Oral Surg Oral Med Oral Pathol 70:286–293
Carrega G, Castagnola E, Canessa A, Argenta P, Haupt R, Dini G, Garaventa A (1994) Herpes simplex virus and oral mucositis in children with cancer. Support Care Cancer 2:266–269
Gomez RS, Carneiro MA, Souza LN, Victória JM, de Azevedo WM, De Marco L, Kalapothakis E (2001) Oral recurrent human herpes virus infection and bone marrow transplantation survival. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91:552–556
Mendonça RMH, Araújo M, Levy CE, Morari J, Silva RA, Yunes JA, Brandalise SR (2011) Prospective evaluation of HSV, Candida spp., and oral bacteria on the severity of oral mucositis in pediatric acute lymphoblastic leukemia. Support Care Cancer. doi:10.1007/s00520-011-1190-0
Bubley GJ, Chapman B, Chapman S, Crumpacker C, Schnipper L (1989) Effect of acyclovir on radiation and chemotherapy induced mouth lesions. Antimicrob Agents Chemother 33:862–865
Redding S, Luce E, Boren M (1990) Oral herpes simplex virus infection in patients receiving head and neck radiation. Oral Surg Oral Med Oral Pathol 69:578–580
Oakley C, Epstein JB, Sherlock CH (1997) Reactivation of oral herpes simplex virus. Implications for clinical management of herpes simplex virus recurrence during radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 84:272–278
Nicolatou-Galitis O, Dardoufas K, Markoulatos P, Sotiropoulou-Lontou A, Kyprianou K, Kolitsi G, Pissakas G, Skarleas C, Kouloulias V, Papanicolaou V, Legakis NJ, Velegraki A (2001) Oral pseudomembranous candidiasis, herpes simplex virus-1 infection, and oral mucositis in head and neck cancer patients receiving radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF) mouthwash. J Oral Pathol Med 30:471–480
Nicolatou-Galitis O, Athanassiadou P, Kouloulias V, Sotiropoulou-Lontou A, Dardoufas K, Polychronopoulou A, Gonidi M, Kyprianou K, Kolitsi G, Skarleas C, Pissakas G, Papanikolaou IS, Kouvaris J (2006) Herpes simplex virus-1 (HSV-1) infection in radiation-induced oral mucositis. Support Care Cancer 14:753–762
Elad S, Zadik Y, Hewson I, Hovan A, Correa MEP, Logan R, Elting LS, Spijkervet FKL, Brennan MT (2010) A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea. Support Care Cancer 18:993–1006
World Health Organization (1979) WHO handbook for reporting results of cancer treatment. World Health Organization, Geneva
Ansar AS, Penhale WJ, Talal N (1985) Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol 121:531–551
Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC (2009) Stronger inflammatory/cytotoxic T cell response in women identified by microarray analysis. Genes Immun 10:509–516
Barasch A, Peterson DE (2003) Risk factors for ulcerative oral mucositis in cancer patients: unanswered questions. Oral Oncol 39:91–100
Vera-Llonch M, Oster G, Hagiwara M, Sonis S (2006) Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma risk factors and clinical consequences. Cancer 106:329–336
Cowan FM, French RS, Mayaud P, Gopal R, Robinson NJ, Oliveira SA, Faillace T, Uusküla A, Nygård-Kibur M, Ramalingam S, Sridharan G, Aouad RE, Alami K, Rbai M, Sunil-Chandra NP, Brown DW (2003) Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sex Transm Infect 79:286–290
Greenberg M, Cohen S, Boosz B, Friedman H (1987) Oral herpes simplex virus infections in patients with leukemia. J Am Dent Assoc 114:483–486
Clemens SAC, Farhat CK (2010) Seroprevalence of herpes simplex 1–2 antibodies in Brazil. Rev Saude Publica 44(4):726–734
Sonis ST (2002) The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with antineoplastic therapy. Crit Rev Oral Biol Med 13:380–389
Epstein JB, Gorsky M, Hancock P, Peters N, Sherlock CH (2002) The prevalence of herpes simplex virus shedding and infection in the oral cavity of seropositive patients undergoing head and neck radiation therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 94:712–716
Conflicts of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Correia, A.V.L., Coêlho, M.R.C.D., de Oliveira Mendes Cahú, G.G. et al. Seroprevalence of HSV-1/2 and correlation with aggravation of oral mucositis in patients with squamous cell carcinoma of the head and neck region submitted to antineoplastic treatment. Support Care Cancer 23, 2105–2111 (2015). https://doi.org/10.1007/s00520-014-2558-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2558-8