Abstract
Purpose
The purpose of the study is to investigate the usefulness of the triplet regimen comprising aprepitant, palonosetron, and dexamethasone in patients treated with highly emetogenic chemotherapy (HEC) and moderately emetogenic chemotherapy (MEC).
Methods
Patients with lung cancer (aged 65.8 ± 8.4 years) who received carboplatin-based MEC and those treated with cisplatin-based HEC were enrolled. The antiemetic regimen for both types of chemotherapy consisted of aprepitant, palonosetron, and dexamethasone based on the May 2010 guidelines prepared by the Japan Society of Clinical Oncology. The incidence of chemotherapy-induced nausea and vomiting (CINV) and the use of salvage treatment were assessed. The primary endpoints were the percentage of patients with a complete response (CR: no nausea and no salvage treatment) during the entire study period (5 days) after chemotherapy, during the acute phase (day 1), and during the delayed phase (days 2–5).
Results
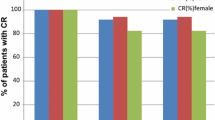
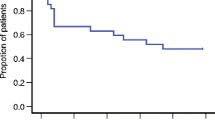
CR rates for the entire period were 86 and 71 % in patients receiving carboplatin-based and cisplatin-based chemotherapy, respectively. CR rates were respectively 98 and 100 % in the acute phase versus 87 and 71 % in the delayed phase. Most of the patients could ingest food throughout the entire period after chemotherapy. Assessment of various risk factors for acute and delayed CINV (gender, age, prior vomiting due to antineoplastic therapy, prior experience of motion sickness, and history of drinking) revealed no significant influence of these factors on the CR rate for the entire period in patients receiving either carboplatin-based or cisplatin-based chemotherapy.
Conclusion
The present triple therapy can be recommended for supporting both carboplatin-based and cisplatin-based chemotherapy regimens.


Similar content being viewed by others
References
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24:4472–4478
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH, American Society of Clinical Oncology (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29:4189–4198
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D, ESMO/MASCC Guidelines Working Group (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(Suppl 5):v232–243
Takahashi T, Hoshi E, Takagi M, Katsumata N, Kawahara M, Eguchi K (2010) Multicenter, phase II, placebo-controlled, double-blind, randomized study of aprepitant in Japanese patients receiving high-dose cisplatin. Cancer Sci 101:2455–2461
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T, Mitsuhashi S (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10:115–124
Warr DG, Grunberg SM, Gralla RJ, Hesketh PJ, Roila F, Rd W, Carides AD, Taylor A, Evans JK, Horgan KJ (2005) The oral NK(1) antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: Pooled data from 2 randomised, double-blind, placebo controlled trials. Eur J Cancer 41:1278–1285
Rapoport BL, Jordan K, Boice JA, Taylor A, Brown C, Hardwick JS, Carides A, Webb T, Schmoll HJ (2010) Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Cancer 18:423–431
Uchino J, Hirano R, Tashiro N, Yoshida Y, Ushijima S, Matsumoto T, Ohta K, Nakatomi K, Takayama K, Fujita M, Nakanishi Y, Watanabe K (2012) Efficacy of aprepitant in patients with advanced or recurrent lung cancer receiving moderately emetogenic chemotherapy. Asian Pac J Cancer Prev 13:4187–4190
Majem M, Moreno ME, Calvo N, Feliu A, Pérez J, Mangues MA, Barnadas A (2011) Perception of healthcare providers versus patient reported incidence of chemotherapy-induced nausea and vomiting after the addition of NK-1 receptor antagonists. Support Care Cancer 19:1983–1990
Hatake K (2011) Risk management in ambulatory anti-cancer therapy, focusing on nausea and vomiting. Gan To Kagaku Ryoho (Cancer Chemother) 38:1758–1760 (in Japanese with English abstract)
Roila F, Hesketh PJ, Herrstedt J, Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (2006) Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 17:20–28
Navari RM (2003) Pathogenesis-based treatment of chemotherapy-induced nausea and vomiting–two new agents. J Support Oncol 1:89–103
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitazaki, T., Fukuda, Y., Fukahori, S. et al. Usefulness of antiemetic therapy with aprepitant, palonosetron, and dexamethasone for lung cancer patients on cisplatin-based or carboplatin-based chemotherapy. Support Care Cancer 23, 185–190 (2015). https://doi.org/10.1007/s00520-014-2339-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2339-4