Abstract
Purpose
Febrile neutropenia (FN) is a common and serious complication of myelosuppressive chemotherapy. Guidelines recommend primary granulocyte colony-stimulating factors (G-CSF) prophylaxis (PPG) in patients with a high risk (HR, >20 %) of developing FN. We performed a retrospective analysis using a subset of the Medicare 5 % database to assess patterns of G-CSF use and FN occurrence among elderly cancer patients receiving myelosuppressive chemotherapy.
Methods
Chemotherapy courses for patients aged 65+ years were identified; only the first course was used for this analysis. Using clinical guidelines, chemotherapy regimens were classified as HR or intermediate risk (IR) for FN. The first administration of G-CSF was classified as either PPG (within the first 5 days of the first cycle), secondary prophylaxis, or reactive.
Results
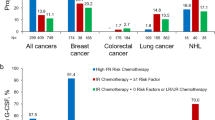
Twelve thousand seven hundred seven courses across five tumor types were classified as having a HR or IR regimen. G-CSF was used in 24.5–73.8 % of patients receiving a HR FN regimen, with the highest use in breast cancer or NHL. Except for breast cancer (where PPG was used in 52.1 %), PPG was given in less than half of patients receiving a HR regimen. Depending on the tumor type, 4.8–22.6 % of patients with a HR regimen had a neutropenia-related hospitalization.
Conclusions
Guidelines recommend PPG with HR FN regimens and older age (>65 years), an important risk factor for developing severe neutropenic complications. However, our results show that in this elderly population, PPG was not routinely used (range 4.8–52.1 %) in patients receiving HR FN regimens. Careful attention to FN risk factors, including chemotherapy regimen and patient age, is needed when planning treatment strategies.

Similar content being viewed by others
References
Kuderer NM, Dale DC, Crawford J, Lyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25:3158–3167
Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J (2010) Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 116:5555–5563
National Comprehensive Cancer Network. NCCN Clinical Practice Guideline in Oncology: Myeloid Growth Factors. V1.2012
Lyman GH, Kuderer NM, Crawford J, Wolff DA, Culakova E, Poniewierski MS, Dale DC (2011) Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 117:1917–1927
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:3187–3205
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32
National Comprehensive Cancer Network. NCCN Clinical Practice Guideline in Oncology: Senior Adult Oncology. V2.2012
Du XL, Lairson DR, Begley CE, Fang S (2005) Temporal and geographic variation in the use of hematopoietic growth factors in older women receiving breast cancer chemotherapy: findings from a large population-based cohort. J Clin Oncol 23:8620–8628
Morrison VA, Wong M, Hershman D, Campos LT, Ding B, Malin J (2007) Observational study of the prevalence of febrile neutropenia in patients who received filgrastim or pegfilgrastim associated with 3–4 week chemotherapy regimens in community oncology practices. J Manag Care Pharm 13:337–348
Tan H, Tomic K, Hurley D, Daniel G, Barron R, Malin J (2011) Comparative effectiveness of colony-stimulating factors for febrile neutropenia: a retrospective study. Curr Med Res Opin 27:79–86
Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG (2006) Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother 40:402–407
Weycker D, Malin J, Barron R, Edelsberg J, Kartashov A, Oster G (2012) Comparative effectiveness of filgrastim, pegfilgrastim, and sargramostim as prophylaxis against hospitalization for neutropenic complications in patients with cancer receiving chemotherapy. Am J Clin Oncol 35:267–274
Heaney ML, Toy EL, Vekeman F, Laliberte F, Dority BL, Perlman D, Barghout V, Duh MS (2009) Comparison of hospitalization risk and associated costs among patients receiving sargramostim, filgrastim, and pegfilgrastim for chemotherapy-induced neutropenia. Cancer 115:4839–4848
Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT (2005) Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 103:1916–1924
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Chen-Hardee S, Chrischilles EA, Voelker MD, Brooks JM, Scott S, Link BK, Delgado D (2006) Population-based assessment of hospitalizations for neutropenia from chemotherapy in older adults with non-Hodgkin’s lymphoma (United States). Cancer Causes Control 17:647–654
Du XL, Osborne C, Goodwin JS (2002) Population-based assessment of hospitalizations for toxicity from chemotherapy in older women with breast cancer. J Clin Oncol 20:4636–4642
Gruschkus SK, Lairson D, Dunn JK, Risser J, Du XL (2010) Comparative effectiveness of white blood cell growth factors on neutropenia, infection, and survival in older people with non-Hodgkin’s lymphoma treated with chemotherapy. J Am Geriatr Soc 58:1885–1895
Gruschkus SK, Lairson D, Dunn JK, Risser J, Du XL (2011) Cost-effectiveness of white blood cell growth factor use among a large nationwide cohort of elderly non-Hodgkin’s lymphoma patients treated with chemotherapy. Value Health 14:253–262
Rajan SS, Stearns SC, Lyman GH, Carpenter WR (2011) Effect of primary prophylactic G-CSF use on systemic therapy administration for elderly breast cancer patients. Breast Cancer Res Treat 130:255–266
Repetto L, Biganzoli L, Koehne CH, Luebbe AS, Soubeyran P, Tjan-Heijnen VC, Aapro MS (2003) EORTC Cancer in the Elderly Task Force guidelines for the use of colony-stimulating factors in elderly patients with cancer. Eur J Cancer 39:2264–2272
American Society of Clinical Oncology (1994) Recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol 12:2471–2508
Lyman GH, Lyman CH, Agboola O (2005) Risk models for predicting chemotherapy-induced neutropenia. Oncologist 10:427–437
Baker J, McCune JS, Harvey RD 3rd, Bonsignore C, Lindley CM (2000) Granulocyte colony-stimulating factor use in cancer patients. Ann Pharmacother 34:851–857
Potosky AL, Malin JL, Kim B, Chrischilles EA, Makgoeng SB, Howlader N, Weeks JC (2011) Use of colony-stimulating factors with chemotherapy: opportunities for cost savings and improved outcomes. J Natl Cancer Inst 103:979–982
Swanson G, Bergstrom K, Stump E, Miyahara T, Herfindal ET (2000) Growth factor usage patterns and outcomes in the community setting: collection through a practice-based computerized clinical information system. J Clin Oncol 18:1764–1770
Acknowledgments
The authors thank Adrine Chung (Chronic Disease Research Group) for project management. This study was supported by a research contract from Amgen Inc, Thousand Oaks, CA
Conflict of interest
CS and TJA are employed by the Chronic Disease Research Group. MRC, VMC, JHP, and RB are employed by and are shareholders of Amgen Inc. AHB reports no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, M.R., Solid, C.A., Chia, V.M. et al. Granulocyte colony-stimulating factor (G-CSF) patterns of use in cancer patients receiving myelosuppressive chemotherapy. Support Care Cancer 22, 1619–1628 (2014). https://doi.org/10.1007/s00520-014-2121-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2121-7