Abstract
Background
The PERFORM Questionnaire is a 12-item scale developed for assessing fatigue in cancer patients in the clinical practice. It has advantages over other tools in that it is short and includes beliefs and attitudes of patients about fatigue. It was psychometrically validated in cancer patients with and without anemia.
Purpose
We evaluated the usefulness of the PERFORM scale to measure fatigue in a large study focusing exclusively on anemic patients.
Methods
This was an observational, multicenter, prospective, 3-month study in cancer patients with hemoglobin (Hb)≤11 g/dl. Fatigue was assessed using the PERFORM questionnaire. The overall score ranges from 12 (no fatigue) to 60 (maximum fatigue).
Results
We included 667 patients: 54.1 % women, mean age 60 (standard deviation, 12) years. A highly significant, but mild correlation was observed between low baseline Hb and high patient perception of fatigue (r with PERFORM score=−0.215, p < 0.0001). Of the patients, 65.8 % improved Hb level during follow-up (increase of ≥1 g/dL and/or achieving >11 g/dL), which translated into a significant improvement in the PERFORM score [mean (95 % confidence interval (CI)] change, −1.2 (−0.04 to −2.4), whereas more fatigue was observed in patients without improvement in Hb [change (95 % CI) in PERFORM, +3.3 (1.5 to 5)]. In a multivariate linear regression analysis, the independent factors associated to fatigue at 3 months were a low Hb level, a low Karnofsky index, active chemotherapy, cancer treatment with palliative intention, and transfusion need in the last 3 months.
Conclusions
Minimal increases or decreases in Hb of ≥1 g/dL were associated with meaningful changes in patient-perceived fatigue as measured with the PERFORM questionnaire. In addition to anemia severity, other factors such as active chemotherapy and advanced disease contribute to perception of fatigue by cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatigue is one of the cancer symptoms with greatest impact in the patients’ daily lives, and it is gaining importance as outcome measure [1]. In a recent survey, it was found that 80 % of patients experienced fatigue at least 50 % of the time during treatment, and fatigue was ranked as the symptom most impacting daily life, independently of gender or tumor type [2]. The underlying pathophysiology of cancer-related fatigue is very complex and not completely understood [3]. Although some common mechanisms seem to participate, it is probable that the etiology is not the same in all cancer subpopulations (i.e., patients undergoing active chemotherapy, patients with advanced age, survivors or those with palliative care). It is very important to correctly identify both the biological and psychosocial determinants of fatigue, in order to individualise the therapeutic management.
The etiology of cancer-related fatigue is multifactorial and is related to a variety of factors including chemotherapy with alkylating agents, antimetabolites or platinum compounds, radiotherapy or bone marrow transplantation, changes in blood volume, excess lactate production, hypoglycemia, hypotension, generalized stress responses with or without endocrine dysfunction, sleep disturbances, anxiety, or depression [4–9]. Anemia is one of the factors identified as a causative element in the fatigue experience [10]. Several studies have demonstrated an association between the hemoglobin (Hb) level or its change over time and fatigue intensity assessed by means of Linear Analogue Self-Assessment (LASA) and visual analogue scale (VAS) [11–13]. Furthermore, an association between low hemoglobin and impaired quality of life has also been observed [14].
Anemia is a common complication in cancer patients. According to the European Cancer Anaemia Survey (ECAS), it is present in 72 % of non-solid and 62 % of solid tumor patients [15]. The underlying causes of low Hb levels include chemotherapy with platinum salts, which affect erythropoietin (EPO) production secondarily to nephrotoxicity [16], direct bone marrow damage, caused by almost all cytotoxic drugs [17–19], or also the underlying malignancy itself, which directly decreases erythropoiesis due to an attenuated endogenous EPO response. Chronic anemia of cancer is also characterized by generalized hypoxia, which results in severe fatigue [20].
Other proposed mechanisms of cancer-related fatigue are a dysregulation of the immune function, a dysfunction of the hypothalamic–pituitary–adrenal axis, altered central nervous system serotonin neurotransmitter activity, vagal afferent signaling, and alterations in muscle metabolism [3]. Several findings support these hypotheses. For example, elevated levels of inflammatory cytokines [21], circulating T lymphocytes [22], increased neutrophil counts [23], and blunted cortisol responses [24, 25] have been found to be associated to fatigue in cancer patients. Other studies have identified associations with sleep disturbances [26–28].
The PERFORM questionnaire is a brief, 12-items scale which was developed under the auspice of the Spanish Society of Medical Oncology. In the validation study performed in 437 patients with and without anemia, it demonstrated good psychometric properties (overall Cronbach’s alpha and intraclass correlation coefficient of 0.94 and 0.83, respectively; effect size of 0.57 for improved patients and −1 for worsened patients; minimally important difference of 3.5) and accurately reflected improvements in Hb levels [29]. Thus, the PERFORM questionnaire constitutes a good tool to assess perceptions of fatigue of cancer patients in the clinical practice. In order to continue our observations, we have performed a large study focusing exclusively on anemic patients.
The main objective was to prospectively evaluate the association between the Hb level (and its change over time) and self-perceived fatigue and quality of life in cancer patients with anemia, controlling by possible confounding factors (age, gender, tumor type and stage, cancer treatments, presence of comorbidities such as anxiety, depression, malnutrition, sleep disorders, etc.). All these factors were collected, and its effect on fatigue was evaluated simultaneously with the effect of Hb level by means of a multivariate analysis, which allowed the estimation of the independent effects. The secondary objectives were to describe anemia management in the clinical practice of Spanish oncology services and to search other clinical, biochemistry, or sociodemographic factors associated to cancer-related fatigue.
Patients and methods
Study design and population
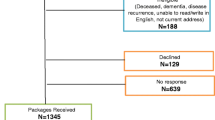
We performed a prospective, multicenter, observational, 3-month study between September 2007 and July 2008 in medical oncology or palliative care departments of 60 Spanish hospitals. The inclusion criteria were: ambulatory patients ≥18 years of age; with a diagnosis of cancer (any site and length of disease duration); with life expectancy of at least 6 months; and with anemia (symptomatic or asymptomatic) defined as Hb ≤11 g/dl, on inclusion. All eligible subjects who fulfilled the inclusion criteria and provided informed consent were consecutively enrolled in the study. The sample size was calculated based on having 80 % power to detect as significant, at a probability of type I error (alpha) of 0.05, a correlation coefficient of at least 0.1 between longitudinal changes in PERFORM overall score and Hb levels. The calculated number of patients required was 660. The study was approved by the Ethics Committee of the Hospital Clínic in Barcelona and has therefore been performed in accordance with the ethical standards laid down in the Declaration of Helsinki.
Variables and procedures
Sociodemographic and clinical characteristics, and hematology (hemoglobin, hematocrit, leucocyte, lymphocytes, neutrophils, platelets), biochemistry (sodium, potassium, aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose, albumin, bilirubin, creatinine, lactate-dehydrogenase (LDH), serum iron, ferritin, transferrin, vitamin B12, folic acid, endogenous EPO), and fatigue measures were collected at baseline visit and 3 months later. Patient perception of fatigue was assessed using the PERFORM questionnaire and two additional instruments, included as control measures: the LASA scale and a VAS.
The PERFORM Questionnaire (Appendix) is a recently developed questionnaire for assessing patient perception on cancer-related fatigue [29, 30]. After the generation of a “pool” of 75 candidate items [31], they were administered to a sample of oncology patients in the preliminary assessment study, conducted between January and September 2005 [30]. The psychometric properties of the final, 12-item version were assessed in the validation study, conducted between November 2005 and September 2006, which showed good feasibility, internal consistency, test–retest reliability, convergent validity, and sensitivity to change [29]. The 12 items, whose responses are on a five-point Likert scale, are distributed in three dimensions “Physical Limitations,” “Activities of Daily Living,” and “Beliefs and Attitudes.” An overall score (range from 12 (no fatigue) to 60 (maximum fatigue)) and three dimension subscores (range from 4 to 20) are obtained, with high scores indicating worse patient perception of cancer-related fatigue.
The LASA scale, previously used for assessing health-related quality of life in cancer patients [12, 32], consists of three items (range for each one from 0 (worse quality of life (QoL)) to 100 (best QoL)): energy scale, activities of daily living, and overall QoL. Each of them identifies a relevant dimension in the evaluation of quality of life in cancer patients. The LASA scale correlates well with Hb levels and has shown good reproducibility and sensitivity to change, with minimally important differences of 9.6 for energy level, 8.7 for activities of daily living, and 9.8 for overall QoL [12]. Finally, each patient self-rated fatigue intensity on a 100-mm horizontal VAS.
Statistical analysis
Correlations between Hb level and patient perception of fatigue at baseline, and between changes in Hb level and changes in fatigue scores during the follow-up were evaluated by using Spearman rank correlation tests. Since the fatigue measures had a normal distribution (confirmed with the Kolmogorov–Smirnov test), changes along time were analyzed using paired T tests in the overall sample and in subgroups of patients defined by an improvement or not in their Hb level during the study (increase of ≥1 g/ dL or achieving >11 g/dL). Mean changes and baseline values between subgroups were compared using Mann–Whitney tests or Student’s T tests (as applicable). The sensitivity to change was assessed by calculating the effect size (i.e., the standardised mean score change) in the subgroups of patients with and without improvement in Hb levels. Changes in hematology and serum biochemistry parameters between baseline and 3-month visits were evaluated using paired T tests.
Bivariate associations between patients’ characteristics (sociodemographic and clinical variables, hematology and biochemistry values) and fatigue measures were assessed using Student’s T tests or analysis of variance. Effect measures were expressed as difference in means together with the 95 % confidence interval with respect to the reference category (the one with less fatigue). A multivariate linear regression model was built to identify the independent factors associated to perception of fatigue (overall PERFORM score) at 3 months. Statistical analyses were performed with the SAS® package version 8.2. (SAS Institute, Cary, NC, USA).
Results
Demographic and clinical characteristics
The study included 667 cancer patients with anemia. The main characteristics of the study population are shown in Table 1.
Hematology and serum biochemistry parameters where mostly within normal limits (Table 2), both at baseline and 3-month visit, with the exception of: low hematocrit and Hb levels (as defined by protocol), and elevated mean LDH and endogenous EPO levels. Ferritin levels were in the upper limit of normality. During the prospective follow-up, the Hb (p < 0.001), hematocrit (p < 0.001), serum iron (p = 0.026), AST (p = 0.002), and albumin levels (p < 0.001) displayed a significant increase, whereas the platelet count (p < 0.001) and the glucose levels (p = 0.007) decreased (Table 2).
Evolution and management of anemia
At baseline, 65.1, 33.3, and 1.6 % of patients had mild (>10 to ≤11 g/dL), moderate (≥8 to ≤10 g/dL), and severe anemia (<8 g/dL), respectively (Table 3). At 3 months, the percentage of anemic patients had decreased to 43.3 %, and 65.8 % of patients had an improvement in their Hb level (defined as increase of ≥1 g/dL or achieving >11 g/dL). The severity of anemia in the subgroup of patients who remained with Hb <11 g/dL was similar than at baseline (Table 3).
Only 42.4 % of patients received treatment for anemia at baseline visit, mainly erythropoiesis-stimulating agents (ESAs) and/or supplements (87 % iron supplementation; Table 3). At 3-month visit, the percentage of patients with treatment for anemia had increased to 55.8 % (p < 0.001), but the relative distribution of the different treatments was similar (Table 3).
Patients treated with transfusions alone or in combination had lower mean baseline Hb level (9.1 (standard deviation (SD), 1.0) g/dL) than any other group (10.0 (SD, 0.7) g/dL in patients with ESA alone, 9.8 (SD, 0.7) g/dL in patients with ESA and supplements, 10.2 (SD, 0.7) g/dL in patients with supplements alone, and 10.3 (SD, 0.6) g/dL in patients without treatment, p < 0.0001 between groups). Mean change in Hb during the follow-up was similar in all these subgroups (p = 0.511, data not shown).
Fatigue measures and correlation with Hb
Table 3 displays mean fatigue scores at baseline and 3-month visits in the overall group for the three administered instruments. At baseline, both the PERFORM questionnaire and the two control measures (LASA and VAS) reflected an impairment of medium-degree intensity. Mean PERFORM overall score at baseline in patients with mild, moderate, and severe anemia was 31.6 (SD, 12.5), 36.6 (SD, 13.9), and 41.6 (SD, 11.9), respectively.
At 3 months, a significant improvement in fatigue was observed as measured by the “Beliefs and attitudes” dimension of the PERFORM questionnaire (p = 0.036) and by the three LASA subcales (p = 0.006, 0.003, and 0.004, respectively; Table 3).
At baseline, the correlations between fatigue measures and Hb level were statistically significant in all cases, and showed relationships of moderate degree (r = −0.215, −0.187, −0.221, −0.164, p < 0.001 in all cases, for the PERFORM overall, activities of daily living, beliefs and attitudes, and physical limitations scores; r = −0.134, p < 0.001 for the VAS score; and r = 0.108, p = 0.005, r = 0.099, p = 0.01 and r = 0.103, p = 0.007 for the energy, activities of daily living, and overall QoL LASA subscales, respectively; Table 4).
At 3 months, the correlation coefficient between Hb level and PERFORM overall score was similar than at baseline visit (r = −0.249, p < 0.0001). A significant negative correlation was also observed between 3-month changes in Hb level and 3-month changes in PERFORM score (r = −0.255, p < 0.0001).
Fatigue in subgroups with and without improvement in Hb
Table 5 shows 3-month changes in fatigue scores in patients with and without improvement in Hb level. At baseline, no significant differences were observed between these two groups.
During the follow-up, mean changes in fatigue scores were significantly different for the three questionnaires, resulting in effect sizes ranging from 0.07 to 0.20 (absolute values) in improved patients, and from 0.12 to 0.32 in non-improved patients (Table 5).
Factors associated to fatigue
At baseline visit, factors associated to fatigue as measured by overall PERFORM score were: Hb level (r = −0.215, p < 0.0001), low Karnofsky score (r = −0.275, p < 0.0001), high ferritin levels (r = 0.338, p = 0.040), need for caregiver (difference in mean versus patients who do not need care, +8.7, p < 0.0001), treatment with palliative intention (+5.2 versus treatment with curative intention, p = 0.001), and metastatic tumor (+4.3 versus local tumor, p = 0.018). No relationship was found with age, gender, educational level, serum iron, transferrin saturation, time since diagnosis, tumor location or active cancer treatment (data not shown).
At 3 months, factors associated to fatigue were: longer time since diagnosis, lower Karnofsky index, lower Hb at baseline and at 3 months, lower change in Hb during the follow-up, lower serum iron at baseline, lung cancer type, metastatic tumor, active cancer treatment, chemotherapy administration, palliative cancer treatment intention, heart and/or respiratory failure, administration of ESA, and transfusion use (Table 6).
After multivariate adjustment, the independent factors that remained associated to fatigue (global PERFORM score) at 3 months were: a low Hb level (coefficient β +1.43 (+0.60 to +2.26) for each −1 g/dL, p = 0.001), a low Karnofsky index (coefficient β +0.19 (+0.08 to +0.29) for each +1 %, p < 0.0001), active chemotherapy (coefficient β 5.07 (+1.81 to +8.32), p = 0.002), cancer treatment with palliative intention (coefficient β +2.97 (+0.33 to +5.61), p = 0.028), and transfusion need in the last 3 months (coefficient β +4.66 (+0.34 to +8.98), p = 0.035).
Discussion
The PERFORM questionnaire is a brief, recently validated scale, specifically developed in the Spanish cultural environment, for the assessment of perceptions and beliefs about cancer-related fatigue, which has demonstrated excellent psychometric properties [30]. The present study constitutes the first use of this 12-item tool in the routine management of Spanish cancer patients.
At baseline, we administered the PERFORM questionnaire to a cohort characterized by low Hb levels, with the aim to describe the relationship between anemia and perception of fatigue by patients. The significant correlation found between the Hb level and the three fatigue measures (the PERFORM questionnaire and the two control measures LASA and VAS), ranging between 0.10 and 0.22, is in line with what has been published so far in the literature [12]. During the follow-up, approximately 50 % of patients had their anemia corrected, and the PERFORM questionnaire was able to capture an improvement in fatigue perception, whereas the LASA scale did not show a significant change. In patients without Hb improvement, an overall worsening of fatigue and QoL was observed, suggesting that patient’s overall status had deteriorated due to cancer progression. In the multivariate analysis, a decrease in Hb levels as little as 1 g/dL was independently associated to a worsening of fatigue perception, after controlling by demographic and clinical characteristics, cancer and anemia treatments, and other biochemistry values. Thus, our results suggest that 1 g/dL is a noteworthy change for the patient’s point of view, at least in anemic patients. In a study with patients in palliative care, differences in fatigue were observed only between subgroups defined by a cutoff level of 10 g/dL, but not when the cutoff level was set to 12 g/dL [33]. We noticed a similar degree of correlation between fatigue and absolute Hb levels at baseline and at 3 months, after anemia correction, which suggests that further benefits can be observed in patient-reported fatigue at levels above 11 g/dl. A previous study of anemia correction with epoetin alfa reported that the greatest QoL increase was recorded when patients approached an Hb level of 12 g/dL, independent of the baseline Hb level [13].
Regarding the management of anemia in the study sample, it is similar than that described in previous studies [15], although we found a slightly higher use of iron supplementation than in the ECAS. Besides Hb level, other factors associated to fatigue in our cohort were the presence of advanced disease (as indicated by the significance of the variable “palliative cancer treatment intention”), chemotherapy administration, and a low Karnofsky index. The relationship found with transfusion need could be explained by the fact that transfusions have only transient effects, and have a limited capacity to ameliorate the symptoms of anemia [34, 35].
We found no significant relationship between fatigue and age, as previously described [36]. Gerber et al. [14], in a cohort of breast cancer patients, found that the biological characteristics associated with fatigue were high body mass index and white blood cell counts. Other studies in breast and liver cancer [37, 38] and in patients with palliative care [33] found that fatigue was associated to psychological factors such as distress, depression, or anxiety (less prevalent in our sample). Through the results of the present and other recent studies, it is becoming evident that cancer-related fatigue has several causal mechanisms [14]. For this reason, an individualised approach to its therapeutic management is needed [39–46].
The present study supports the potential usefulness of the brief PERFORM questionnaire for quickly assessing patient perception of fatigue in the clinical practice. A systematic review conducted in 2009 identified an extremely high number (up to 40) of validated instruments for measuring fatigue in cancer patients [47]. Only few of them were optimally tested for validity and reliability [48], and most tools were relatively insensitive to differences in fatigue to cancer stage. In addition, most instruments were too long to be administered in patients with advanced cancer. In the present study, the PERFORM questionnaire, which is almost as short as the nine-item Brief Fatigue Inventory, has demonstrated good sensitivity to change, and has the advantage over other tools that includes beliefs and attitudes of patients about fatigue. It is possible that this newly added dimension explains our findings about factors predicting fatigue not found in any previous study (i.e., transfusion need).
The main limitation of the study is the observational design, which does not allow the establishment of causal relationships. Treatment bias does not allow comparing outcomes in Hb or fatigue between subgroups defined by therapeutic management of anemia. Indirect associations between unmeasured variables and some of the described factors predicting fatigue might account for some of our findings, and thus results must be interpreted with caution.
Conclusions
Minimal increases or decreases in Hb of ≥1 g/dL were associated with meaningful changes in patient-perceived fatigue as measured with the brief, 12-item PERFORM questionnaire. In addition to anemia severity, other factors such as active chemotherapy and advanced disease contribute to perceptions of fatigue by cancer patients. These results represent new evidence of the potential usefulness of PERFORM questionnaire for monitoring symptoms of fatigue in cancer patients.
References
Mock V, Atkinson A, Barsevick A, Cella D, Cimprich B, Cleeland C, Donnelly J, Eisenberger MA, Escalante C, Hinds P, Jacobsen PB, Kaldor P, Knight SJ, Peterman A, Piper BF, Rugo H, Sabbatini P, Stahl C (2000) NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park) 14:151–161
Diaz N, Menjon S, Rolfo C, Garcia-Alonso P, Carulla J, Magro A, Miramon J, Rodriguez CA, de Castellar R, Gasquet JA (2008) Patients’ perception of cancer-related fatigue: results of a survey to assess the impact on their everyday life. Clin Transl Oncol 10:753–757
Barsevick A, Frost M, Zwinderman A, Hall P, Halyard M (2011) I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res 19:1419–1427
Sprangers MA, Van Dam FS, Broersen J, Lodder L, Wever L, Visser MR, Oosterveld P, Smets EM (1999) Revealing response shift in longitudinal research on fatigue: the use of the thentest approach. Acta Oncol 38:709–718
Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH (1999) Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manag 18:233–242
Molassiotis A, Morris PJ (1999) Quality of life in patients with chronic myeloid leukemia after unrelated donor bone marrow transplantation. Cancer Nurs 22:340–349
Berger AM, Farr L (1999) The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncol Nurs Forum 26(10):1663–1671
Romito F, Montanaro R, Corvasce C, Di Bisceglie M, Mattioli V (2008) Is cancer-related fatigue more strongly correlated to haematological or to psychological factors in cancer patients? Support Care Cancer 16:943–946
Yennurajalingam S, Palmer JL, Zhang T, Poulter V, Bruera E (2008) Association between fatigue and other cancer-related symptoms in patients with advanced cancer. Support Care Cancer 16:1125–1130
NCCN (2013) Cancer-related fatigue. NCCN Clinical practice guidelines in oncology. Version 1.2013. Available at: http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf. Accessed 21 Nov 2012
Gabrilove JL, Cleeland CS, Livingston RB, Sarokhan B, Winer E, Einhorn LH (2001) Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol 19:2875–2882
Patrick DL, Gagnon DD, Zagari MJ, Mathijs R, Sweetenham J (2003) Assessing the clinical significance of health-related quality of life (HrQOL) improvements in anaemic cancer patients receiving epoetin alfa. Eur J Cancer 39:335–345
Carteni G, Giannetta L, Ucci G, De Signoribus G, Vecchione A, Pinotti G, Puglisi F, Contillo A, Pezzella G, Orecchia S, Beccaglia P (2007) Correlation between variation in quality of life and change in hemoglobin level after treatment with epoetin alfa 40,000 IU administered once-weekly. Support Care Cancer 15:1057–1066
Gerber LH, Stout N, McGarvey C, Soballe P, Shieh CY, Diao G, Springer BA, Pfalzer LA (2011) Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Support Care Cancer 19:1581–1591
Ludwig H, Van Belle S, Barrett-Lee P, Birgegard G, Bokemeyer C, Gascon P, Kosmidis P, Krzakowski M, Nortier J, Olmi P, Schneider M, Schrijvers D (2004) The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 40:2293–2306
Canaparo R, Casale F, Muntoni E, Zara GP, Della Pepa C, Berno E, Pons N, Fornari G, Eandi M (2000) Plasma erythropoietin concentrations in patients receiving intensive platinum or nonplatinum chemotherapy. Br J Clin Pharmacol 50:146–153
Tas F, Eralp Y, Basaran M, Sakar B, Alici S, Argon A, Bulutlar G, Camlica H, Aydiner A, Topuz E (2002) Anemia in oncology practice: relation to diseases and their therapies. Am J Clin Oncol 25:371–379
Rajeswaran A, Trojan A, Burnand B, Giannelli M (2008) Efficacy and side effects of cisplatin- and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: a systematic review of randomized controlled trials. Lung Cancer 59:1–11
Demetri GD (2001) Anaemia and its functional consequences in cancer patients: current challenges in management and prospects for improving therapy. Br J Cancer 84(Suppl 1):31–37
Ludwig H (1999) Epoetin in cancer-related anaemia. Nephrol Dial Transplant 14(Suppl 2):85–92
Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR (2006) Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 12:2759–2766
Bower JE, Ganz PA, Aziz N, Fahey JL, Cole SW (2003) T-cell homeostasis in breast cancer survivors with persistent fatigue. J Natl Cancer Inst 95:1165–1168
Gripp S, Moeller S, Bolke E, Schmitt G, Matuschek C, Asgari S, Asgharzadeh F, Roth S, Budach W, Franz M, Willers R (2007) Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol 25:3313–3320
Bower JE, Ganz PA, Aziz N (2005) Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med 67:277–280
Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW (2007) Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav Immun 21:251–258
Ancoli-Israel S, Moore PJ, Jones V (2001) The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care (Engl) 10:245–255
Berger AM, Farr LA, Kuhn BR, Fischer P, Agrawal S (2007) Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manag 33:398–409
Roscoe JA, Kaufman ME, Matteson-Rusby SE, Palesh OG, Ryan JL, Kohli S, Perlis ML, Morrow GR (2007) Cancer-related fatigue and sleep disorders. Oncologist 12(Suppl 1):35–42
Baro E, Carulla J, Cassinello J, Colomer R, Mata JG, Gascon P, Gasquet JA, Rodriguez CA, Valentin V (2011) Psychometric properties of the Perform Questionnaire: a brief scale for assessing patient perceptions of fatigue in cancer. Support Care Cancer 19:657–666
Baro E, Carulla J, Cassinello J, Colomer R, Mata JG, Gascon P, Gasquet JA, Herdman M, Rodriguez CA, Sanchez J, Valentin V (2009) Development of a new questionnaire to assess patient perceptions of cancer-related fatigue: item generation and item reduction. Value Health 12:130–138
Baro E, Herdman M, Gasquet J, Sánchez J (2004) Instruments to measure patient-reported outcomes and perceptions of cancer-related fatigue: a review of the literature. Value Health 7:PCN32
Demetri GD, Kris M, Wade J, Degos L, Cella D (1998) Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: results from a prospective community oncology study. Procrit Study Group. J Clin Oncol 16:3412–3425
Munch TN, Zhang T, Willey J, Palmer JL, Bruera E (2005) The association between anemia and fatigue in patients with advanced cancer receiving palliative care. J Palliat Med 8:1144–1149
Ludwig H (2002) Anemia of hematologic malignancies: what are the treatment options? Semin Oncol 29:45–54
Osterborg A (1998) Recombinant human erythropoietin (rHuEPO) therapy in patients with cancer-related anaemia: what have we learned? Med Oncol 15(Suppl 1):S47–S49
Aapro MS, Cella D, Zagari M (2002) Age, anemia, and fatigue. Semin Oncol 29:55–59
Haghighat S, Akbari ME, Holakouei K, Rahimi A, Montazeri A (2003) Factors predicting fatigue in breast cancer patients. Support Care Cancer 11:533–538
Shun SC, Lai YH, Jing TT, Jeng C, Lee FY, Hu LS, Cheng SY (2005) Fatigue patterns and correlates in male liver cancer patients receiving transcatheter hepatic arterial chemoembolization. Support Care Cancer 13:311–317
Mitchell SA (2010) Cancer-related fatigue: state of the science. PM R 2:364–383
Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ (2007) Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol 26:660–667
Luctkar-Flude MF, Groll DL, Tranmer JE, Woodend K (2007) Fatigue and physical activity in older adults with cancer: a systematic review of the literature. Cancer Nurs 30:E35–E45
Revicki DA, Stull D, Vernon M, Rader M, Tomita D, Viswanathan HN (2012) Assessing the effect of darbepoetin alfa on patient-reported fatigue in chemotherapy-induced anemia in four randomized, placebo-controlled clinical trials. Qual Life Res 21:311–321
Glaspy J (2001) Anemia and fatigue in cancer patients. Cancer 92:1719–1724
Esquerdo G, Llorca C, Cervera JM, Orts D, Juarez A, Carrato A (2011) Effectiveness of darbepoetin alfa in a cohort of oncology patients with chemotherapy-induced anaemia. Relationship between variation in three fatigue-specific quality of life questionnaire scores and change in haemoglobin level Clin Transl Oncol 13:341–347
Calabrich A, Katz A (2011) Management of anemia in cancer patients. Future Oncol 7:507–517
De Waele S, Van Belle S (2010) Cancer-related fatigue. Acta Clin Belg 65:378–385
Seyidova-Khoshknabi D, Davis MP, Walsh D (2011) Review article: a systematic review of cancer-related fatigue measurement questionnaires. Am J Hosp Palliat Care 28:119–129
Agasi-Idenburg C, Velthuis M, Wittink H (2010) Quality criteria and user-friendliness in self-reported questionnaires on cancer-related fatigue: a review. J Clin Epidemiol 63:705–711
Acknowledgments
The study was conducted under the auspice of the Spanish Society of Medical Oncology. Writing assistance was supported by Amgen S.A. and provided by Dr. Neus Valveny from Trial Form Support. The authors wish to acknowledge Ariadna Lloansí (Amgen S.A.) for the critical review of the manuscript and Amgen SA for financial support of this study. The conclusions, interpretations, and opinions expressed herein are those of the authors.
PERFORM IV Study Group (in alphabetical order)
Dr. Adolfo Frau Llopis, H. Provincial de Castellón; Dr. Adolfo Murias, Hosp. Insular de Gran Canaria; Dr. Alberto Arizcun, Compl. Hosp. de Palencia (Hosp. Río Carrión); Dr. Alberto Rodriguez, Hosp. Juan Ramón Jiménez; Dra. Almudena Martín, Hosp. Severo Ochoa; Dra. Amalia Velasco Ortiz, Hosp. La Princesa; Dr. Antonio González, Hosp. Ramón y Cajal; Dr. Antonio Sánchez, Dr. Mariano Provencio, Hosp. U. Puerta de Hierro; Dra. Belén Loboff, Dra. M. Luisa Gonzalvez, Hosp. Universitario Virgen de la Arrixaca; Dr. Brunet, Dra. Montse Velazco, ICO de Girona; Dr. Camps, Dra. Ana Blasco, Hosp. Gral. de Valencia; Dr. Carrato, Hosp. Gral. U. de Elche; Dr. César Rodríguez, Hosp. U. de Salamanca; Dra. Dolores Isla, Hosp. Lozano Blesa; Dra. Elena Filipovich, Dra. Juana Mª Cano, Hosp. Ntra Sra. de Sonsoles; Dr. Francisco Ayala, Hosp. Morales Meseguer; Dra. Hae Jin SuhOh, Dr. Manuel Constenla, Hosp. de Pontevedra; Dr. Ignacio Duran, Hosp. de Madrid Norte Sanchinarro; Dr. J. L. Pérez Gracia, Clínica Univ. de Navarra; Dr. Javier Cassinello, Hosp. U. de Guadalajara ; Dr. Javier Castellanos, Dra. Lidia Vázquez, H. Xeral Cíes; Dr. Javier Puertas, Hosp. Río Hortega; Dr. Jesús García Mata, Compl. Hosp. Ourense (Hosp. Sta. María de Naí); Dra. Jiménez Orozco, Hosp. Gral. de Jerez ; Dr. Joan Carulla, Hosp. Mateu Orfila ; Dr. José Manuel Baena, Dr. Antonio Rueda, Hosp. Puerta del Mar; Dr. José Manuel Cuervo, Hosp. San Juan de Dios; Dr. José Ramón Mel, Hosp. Xeral Calde; Dr. Josep Manuel Piera, Hosp. Donostia; Dr. Juan Cueva Bañuelos, Hosp. Cl. Santiago de Compostela; Dr. Juan Ramón Delgado, Hosp. Virgen de las Nieves; Dr. Miguel Angel Cruz, Hosp. Virgen de la Salud; Dr. Miguel Mendez, Hosp. de Móstoles; Dra. Nieves Díaz, Hosp. San Juan de Alicante; Dr. Norberto Batista, Hosp. Univ. de Canarias La Laguna; Dr. Pedro López Tendero, Dra. Dolores Torregrosa, Dra. Regina Girones, Hosp. de Jativa/Xativa; Dr. Pedro Sánchez Rovira, Hosp. Gral. de Jaén; Dr. Pere Gascón, Hosp. Clínic i Provincial; Dra. Pilar García Alfonso, Hosp. Gregorio Marañón; Dr. Puerto Pica, Dr. Álvarez, Hosp. Infanta Cristina; Dra. Purificación Martínez del Prado, Hosp. de Basurto; Dr. Ramón Colomer, Centro MD Anderson; Dr. Ramón Pérez Carrión, Hosp. Quirón; Dra. Raquel Molina Villaverde, Hosp. Principe de Asturias; Dr. Roberto Fernández, Dr. Ignacio Peláez, Hosp. de Cabueñes; Dra. Ruth Vera, Hosp. de Navarra; Dr. Salvador Saura, Hosp. de Gran Canaria; Dr. Vicente Valentí, Hosp. Gral. de Catalunya; Dr. Vicente Valentin, Hosp. 12 de Octubre.
Authorship and disclosures
J Carulla, J Cassinello, RC, JG, PG, CR and VV designed the study, coordinated the group, contributed to clinical data collection and reviewed the analysis and the manuscript. EB reviewed the analysis and the manuscript. All the authors approved the final version of the manuscript.
Eva Baró is employee of 3D Health Research. The authors declare no other conflict of interest relating to the publication of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gascón, P., Rodríguez, C.A., Valentín, V. et al. Usefulness of the PERFORM questionnaire to measure fatigue in cancer patients with anemia: a prospective, observational study. Support Care Cancer 21, 3039–3049 (2013). https://doi.org/10.1007/s00520-013-1862-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1862-z