Abstract
Purpose
This retrospective single-institution cohort study aims to evaluate if therapeutic approach, tumour site, tumour stage, BMI, gender, age and civil status predict body weight loss and to establish the association between weight loss on postoperative infections and mortality.
Methods
Consecutive patients with head and neck cancer were seen for nutritional control at a nurse-led outpatient clinic and followed-up for 2 years after radiotherapy. Demographic, disease-specific and nutrition data were collected from case records. The primary outcome measure was maximum body weight loss during the whole study period.
Results
The nadir of body weight loss was observed 6 months after radiotherapy. In total, 92 patients of 157 (59%) with no evidence of residual tumour after treatment received enteral nutrition. The mean maximum weight loss for patients receiving enteral nutrition and per oral feeding was 13% and 6%, respectively (p < 0.001). Using multivariate analysis, tumour stage (p < 0.001) was the only independent factor of maximum weight loss. Weight loss was not significantly related to risk for postoperative infection.
Conclusions
Weight loss is frequently noted among head and neck cancer patients during and after treatment. Weight loss was not found to be associated with postoperative infections and mortality. Nutritional surveillance is important in all patients, but special attention should be given to those on enteral nutrition and those with more advanced disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the treatment of patients with head and neck (H and N) cancer, nutritional issues have been raised and discussed for decades. The tumour itself, radiation-induced fibrosis and surgical defects are reported to be followed by excessive weight loss and malnutrition [1, 2]. Problems include dysphagia, xerostomia, mucositis, loss of appetite, smell and taste changes, as well as pain and badly fitted dentures [3–5]. Long-term malnutrition is one of the reported sequelae of H and N cancer, possibly related to muscle loss, cachexia and psychological and emotional distress [2, 4]. Greater weight loss during radiotherapy (RT) has been associated with postsurgical infections and wound healing problems [6–8]. Weight loss has also been found to be related to increased mortality in H and N cancer patients [6], but the issue is controversial and debated. It is important to be aware of the fact that nutritional problems are often already present before treatment, which is probably related to the tumour site [9], and that negative lifestyle factors may precede cancer diagnosis [10].
The nutritional management of patients with H and N cancer is very complex because several factors are involved, including the disease itself, treatment modalities and other individual factors. Some studies have reported that cancer patients may benefit from early identification and treatment of poor nutritional status [11, 12]. Regular measurements of body weight during and after treatment of H and N cancer have been identified as an important and cheap method to use for surveillance [13]. In addition, body mass index (BMI) has been suggested as one important indicator, especially in patients receiving RT [14].
The management of prevention and treatment for weight loss is an area of great interest, but a golden standard is still not available. Some authors suggest that the indication for enteral nutritional treatment is weight loss greater than 5% of the patients' initial weight, whereas others advocate that enteral therapy should begin before RT treatment [15, 16]. In most cases, enteral tube feeding is the method of choice for nutritional treatment in H and N cancer patients with swallowing problems because the majority of such patients have normal gastrointestinal absorption [5]. The two most common ways for enteral administration is via a polyurethane nasogastric feeding tube (NGT) or a percutaneous endoscopic gastrostomy (PEG) tube.
A number of factors influence the long-term effects of RT and surgery on body weight loss in H and N cancer patients. However, these factors, including how much body weight patients actually lose, how weight loss affects the risk for postoperative infection, as well as morbidity and mortality, have not been thoroughly and systematically investigated. This study is utilising information from a database with records from a structured nutritional surveillance programme offered to H and N cancer outpatients and led by registered nurses. The purposes of this study were (1) to evaluate if therapeutic approach, tumour site, tumour stage, BMI, gender, age and civil status predict body weight loss and (2) to examine the association between weight loss on postoperative infections and mortality in a cohort of H and N cancer patients during RT and up to 2 years after termination of RT.
Patients and methods
Sample
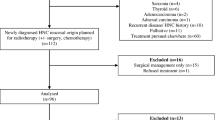
During 2000–2004, 232 consecutive outpatients with H and N cancer were offered nutritional follow-up at a nurse-led outpatient clinic before RT. The patients were diagnosed and treated at the Departments of ENT and Oncology, Karolinska University Hospital, Stockholm, Sweden. Forty-eight patients were neither motivated nor did they attend the nurse-led outpatient clinic for nutritional follow-ups, three died at the beginning of the treatment, two patients were initially planned for preoperative radiotherapy but underwent surgery and postoperative RT and one was excluded because of an initial incorrect diagnosis. The present retrospective study includes the remaining sample of 178 (77%) patients with H and N cancer that agreed to participate in the nutritional follow-ups.
Treatment
Histological specimens of H and N cancer were obtained by endoscopic examination under general anaesthesia. The treatment modality was presented to the patient at a weekly multiprofessional team conference. Treatment consisted of either external beam RT (EBRT) as a single modality treatment or preoperative EBRT followed by surgical excision. The protocol included assessment of therapeutic response using clinical examination or endoscopic control under general anaesthesia about 4 weeks after EBRT. Surgery was generally performed 4 to 6 weeks after termination of EBRT.
Structured nutritional surveillance programme
The patients were followed from the first initial visit at the ENT clinic before treatment. Before treatment, a dietician offered all patients dietary counselling, giving advice about high-caloric diet, and when needed, patients were offered nutritional supplements. The patients' body weight was regularly assessed using two identical waves (patients did not have shoes and outdoor clothing when weighed). Patients with dysphagia and weight loss of >5% of their initial body weight (defined as the weight taken at the initial diagnostic endoscopy) and patients with expected nutritional problems that were caused by advanced tumour (stage IV) were offered enteral nutrition (EN) using a NGT or PEG.
Data collection
All data were collected from nursing and medical records. Data were collected from the first clinical visit and up to 2 years after RT.
Demographic and disease-specific data
Demographic data collected were age, gender and civil status. Disease-specific data were diagnosis, TNM classification and tumour stage, date and treatment plan decided at the weekly multiprofessional team conference, start and end of RT, type of RT, brachytherapy, RT dose given in Grays (Gy), result of RT, major surgery or not, date and type of surgery, patho-anatomical diagnosis (PAD), postoperative infection requiring intravenous or per oral antibiotic treatment (i.e. wound infection, pneumonia or urinary tract infection), recurrence (including cancer progress during RT, residual within 6 months and recurrence after 6 months) and deceased within 2 years.
Nutritional data
BMI was calculated with the following formula: kg/m². BMI 1 ranges from underweight, <20 if <70 years old; if >70 years old, <22; BMI 2 = normal weight >20/22–24.9; BMI 3 = overweight, 25.0–29.9 and BMI 4 = obese, 30.0 and above for sick adults [17]. Body weight: at initial diagnostic endoscopy, at start of RT, after 2 weeks of RT, after 4 weeks of RT, at end of RT, 1 month after RT termination, at the time of surgery, 6 months after termination of RT and 1 to 2 years after RT. Nutritional support: EN or no EN and when nutritional support was given to the patient in relation to the treatment (before, during or after RT).
Statistical analyses
The lowest registered body weight during the whole study period was compared with the first registered pre-treatment body weight and defined as the maximum weight loss expressed in per cent. Data were analysed with regard to treatment, either single modality radiotherapy (RT group) or combined treatment (RT and surgery group). To analyse variances of groups, unpaired t test or one-way ANOVA was used. To analyse the relationship between variables, linear regression was applied to predict maximum weight loss. The variables used in the linear regression analysis were tumour stage (1 = I, 2 = II, 3 = III and 4 = IIII), tumour site (1 = larynx, 2 = oropharynx or oral cavity), surgery (1 = no and 2 = yes), gender (1 = men and 2 = woman) and age (numerical). The study is a retrospective explorative study, and to answer the main objectives regarding predictors of weight loss and the association between weight loss and postoperative infections, the sub-groups of patients were found to be too small and thereby rendering lack of statistical power. However, to detect a significant difference of alpha <0.05 with a power of 0.80 in weight loss between patients receiving EN and those not receiving EN, 49 patients in each group were demonstrated to be enough. Statistical analyses were carried out using GraphPad Prism and SPSS. A p < 0.05 was considered significant. The study was reviewed and approved by the Regional Ethical Board in Stockholm (2005/48-31).
Results
Descriptive data
Table 1 presents the characteristics of the 178 patients (mean age of 60.4 years; range, 29–85 years). The three largest sub-groups were patients with oropharyngeal cancer (n = 73), of which 79% received combined modality treatment, patients with cancer in the oral cavity (n = 42) and patients with laryngeal cancer (n = 37), where 79% and 32%, respectively, received combined modality treatment. To study the difference in weight loss between single modality RT and combined treatment (preoperative RT and surgery), the cohort was stratified according to therapeutic approach. In Table 2, treatment and response for patients receiving RT as single modality treatment (n = 60) are presented. The therapeutic result was controlled with either clinical evaluation (28 patients, 47%) or endoscopic control (32 patients, 53%). Fifty-two patients (87%) showed a clinical complete response and constituted the RT group. Table 3 shows treatment and response for patients receiving combined modality treatment (n = 118). Seventy patients (59%) underwent neck dissection, 15 patients (13%) underwent resection of the primary tumour and 33 patients (28%) underwent resection of the primary tumour and neck dissection with or without reconstruction. Altogether, 105 patients (89%) had radical surgery or no evidence of microscopic tumour after RT and thereby constituted the RT and surgery group.
Weight loss and enteral nutrition
Weight loss during the observation period
Information about the patients' weight and nutritional situation for the two groups is presented in Table 4, showing a nadir of mean body weight 6 months after RT. Twelve patients in the RT group (23%) and 4 patients in the RT and surgery group (4%) retained or gained weight, 11 (21%) and 16 (15%) patients lost <5% of their initial body weight, 9 (17%) and 29 (28%) patients lost between 5% and 10%, 16 (31%) and 44 (42%) patients lost >10% and <20% and 4 (8%) and 12 (11%) patients lost ≥20%, respectively. Of the latter 16 patients with maximum weight loss of ≥20%, 9 had oropharyngeal cancer (stage III = 2 patients, stage IV = 7 patients), 2 had cancer in the oral cavity (stage II = 2 patients), 2 had hypopharyngeal cancer (stage IV = 2 patients), 1 had laryngeal cancer (stage IV = 1 patients), 1 had nasopharyngeal cancer (stage III = 1 patient) and 1 had an unknown primary with cervical lymph node metastasis.
The mean BMI at the time of diagnosis was, for the RT group, 24.9 (SD, 4.7; range, 17–39) and for the RT and surgery group, 25.4 (SD, 3.8; range, 17–37). There was no difference in maximum weight loss between the four BMI groups (BMI calculated from the first registered weight) in the RT group. The mean maximum body weight loss was for BMI 1, 5%; BMI 2, 9%; BMI 3, 6% and BMI 4, 10%. However, in the RT and surgery group, a significant difference was observed (p < 0.05). Mean maximum body weight loss was for BMI 1, 7%; BMI 2, 9%; BMI 3, 13% and BMI 4, 12%.
Weight loss at the 1- to 2-year follow-up period
Altogether, 141 out of 157 patients (90%) were still alive at the 1- to 2-year follow-up period. Of these patients, 122 were considered tumour free, and weight was registered in 81 (66%) of them. The mean body weight loss was 7.5% between the initial weight and the weight taken at the 1- to 2-year follow-up and did not significantly (p = 0.06) differ from the mean body weight loss 6 months after RT (10%). The corresponding figures for the patients with recurrence (the weight registered in 13 of 19 patients, i.e. 68%) were non-significant (p = 0.89) (7% and 8%, respectively).
Enteral nutrition
In the RT group, 21 out of 52 patients (40%) and in the RT and surgery group, 71 out of 105 patients (68%) received EN. Three of them started EN before, 62 during and 27 after termination of RT. Characteristics of patients receiving EN and patients that did not receive EN are shown in Table 5. The mean maximum body weight loss for patients receiving EN (n = 92) and per oral feeding (n = 65) was 13% and 6%, respectively, showing a significant difference (p < 0.001).
Of the 94 (81 tumour free and 13 with recurrence) patients that had registered weight 1 to 2 years after termination of treatment, 60 (64%) had received EN and 34 (36%) had received per oral feeding. Patients that had received EN had lost significantly more weight (10%) than patients with oral feeding (3%) at the 1- to 2-year follow-up period (p < 0.001).
Importance of weight loss for postoperative infection and mortality
Postoperative infections
Thirty-four of the 105 patients (32%) in the RT and surgery group had a postoperative infection. No significant difference was found between the mean preoperative weight loss in patients with postoperative infection (7%) and patients without postoperative infection (6%).
Deceased patients
Within 2 years after RT, 29 patients died; the mean maximum weight loss of these patients was 9%, which can be compared with 10% for the 128 patients that were still alive. No significant difference in maximum body weight loss was found between the two groups.
Predictive factors of maximum body weight loss
There were no significant differences in maximum body weight loss between gender, different age groups (29–49, 50–59, 60–69 and 70–85 years old) and civil status (married/cohabiting and living alone). When the groups diagnosed with cancer in the oropharynx, oral cavity or larynx were compared, there was a significant difference in maximum body weight loss (p < 0.001). The mean body weight loss for patients with cancer of the oropharynx was 11%, for patients with cancer of the oral cavity, 10%, and for patients with cancer of the larynx, 5%. There was also a significant difference for the whole sample when comparing the different tumour stages, I to IV (p < 0.0001). The mean maximum body weight loss for patients with stages I, II, III and IV was 3%, 9%, 10% and 12%, respectively. The mean maximum body weight loss in patients that underwent combined treatment with EBRT and surgery was 11% and was significantly higher than in patients that received only EBRT (7%, p < 0.005).
Linear regression analysis was done with maximum body weight loss as the dependent variable. The independent variables were tumour stage, tumour site, surgery, gender and age. The linear regression analysis showed that only tumour stage was significantly predictive of maximum body weight loss (Table 6). In total, the model explained 19.7% of the variance.
Discussion
This is a unique material from a nurse-led outpatient clinic for nutritional control of H and N cancer patients. At diagnosis, all patients received nutritional counselling and were informed that a high-caloric intake was important in order to avoid weight loss during RT. The patients in general gained weight before the start of RT. Thereafter, the mean weight decreased gradually, reaching a nadir at about 6 months after RT. Patients receiving combined modality treatment had significantly greater maximum weight loss as well as greater weight loss after RT in connection to surgery.
By using nutritional intervention on patients undergoing RT, Isenring et al. [18] showed an improved dietary intake both in energy and protein compared with standard practice. There was also an indication of weight loss in the intervention group. In addition, in a prospective randomised trial, Lee et al. [19] demonstrated the efficacy of nutritional support. Patients receiving follow-up by a special programme that included nutritional counselling and nutritional support had significantly less weight loss compared with a group that did not get such help. It has also been shown that patients receiving EN with an enriched formula had less local wound complications [8]. In our study, a dietician gave all patients nutritional counselling, but the guidelines for tube feeding were based mainly on a wait-and-see procedure in which patients with swallowing problems and loss of >5% of their pre-treatment weight were offered EN through the use of a NGT or PEG. Another established indication for EN was patients with expected nutritional problems caused by advanced tumour (stage IV). It should be emphasised that the literature offers no clear consensus of the optimal method for nutritional management in H and N cancer patients [20]. Some authors recommend elective PEG to all patients before treatment. However, the potential benefit from a wait-and-see procedure with PEG insertion is supported by the findings of complications and prolonged dysphagia in patients receiving PEG [15, 21, 22]. In our study, 87% of patients receiving single modality RT and 89% of patients who underwent combined treatment were considered tumour free after treatment; of these, 56% and 81%, respectively, had a maximum weight loss of more than 5%. A total of 59% received EN. Enteral feeding could not restore weight loss, and patients given tube feeding lost significantly more body weight compared with the group that could maintain adequate oral feeding. This finding is in agreement with results from Nguyen et al. [23] who reported that 98% of 104 H and N cancer patients receiving a PEG before chemoradiation treatment lost weight despite enteral feeding and nutritional assessment from a dietician.
Malnutrition, which is multifactorial in origin, is reported to be a potential source of increased morbidity and mortality in H and N cancer patients [24]. Therefore, optimising nutrition is thought to improve the outcome of treatment as well as affect survival rate. In our study, the clinical significance of moderate weight loss was found to be of modest importance during both treatment and rehabilitation periods. Moreover, 8% and 11% in the RT group and RT and surgery group, respectively, lost ≥20% in weight during the follow-up period. Weight loss was not identified as a risk factor for postoperative infection.
Reduced dietary intake and increased energy expenditure are two main attributes to describe loss of body weight [10]. Deterioration of physical function and performance has been observed when treating H and N cancer patients with chemoradiation. Such deterioration is thought to be due to abnormal changes in metabolism, body composition and the inflammatory state [25]. In the present unselected H and N cancer patient cohort, univariate analysis revealed that therapeutic approach, tumour site and tumour stage were correlated to maximum weight loss. Patients received treatment appropriate for tumour site and stage. Considering this aspect, patients with cancer in the oral cavity or oropharynx and patients receiving combined treatment modality showed the greatest weight loss. However, in the multivariate analysis, the only prognostic predictor was tumour stage. The results are commensurate with findings in a study on H and N cancer patients with stages I and II during EBRT, where tumour site and stage were found to be associated with weight loss [26]. Overall, we found that weight loss did not influence the therapeutic results 1–2 years after termination of treatment. This finding is contrary to that of Pedruzzi et al. [27]. In their study of patients with cancer in the oropharynx, weight loss was shown to be a significant predictor of treatment response for survival.
The WHO recommendation to consider a patient underweight is a BMI <18.5 kg/m². In a study by Isenring et al. [28] of 50 cancer patients prior to RT, only 3 of 50 (6%) were classified underweight with a BMI <18.5 kg/m², which indicates some limitations in using the WHO classification for cancer patients. In our study, we define underweight as a BMI <20 kg/m² in patients <70 years old, and in patients >70 years old, <22 kg/m², in accordance with the Swedish national recommendations for severely sick people [17]. According to this classification, 18 of 157 patients (11%) in our study were classified as underweight before treatment. Hence, by using this wider range to find patients who are at significant risk for malnutrition, a higher number of patients were identified at the time of diagnosis. Only 8 of the 157 patients (5%) would have been classified as underweight if the classification >18.5 kg/m² had been used in the present cohort. Despite the relatively high number of patients underweight before the start of treatment, a low BMI was not found to be a risk factor for weight loss in patients that received RT and surgery.
In the present study, there was a trend for partial recovery of mean body weight for tumour-free patients 1–2 years after termination of RT. At this time of observation, the group of patients receiving enteral feeding still had significantly lost more body weight than the group of patients that could maintain oral feeding. One interpretation of this finding is that enteral feeding as a method to reduce weight loss is not completely successful. This lack of success could depend on a number of factors but nevertheless indicate that patients receiving EN could properly benefit the most from regular follow-ups in a longer perspective after treatment.
The main limitation of the current study is the retrospective and explorative design that does not make it possible to perform adequate power analysis which restricts generalization. However, this is a unique database of a cohort systematically followed-up with regard to weight loss during a long time after treatment. Together with that, H and N cancer patients constitute a small and vulnerable group which is often difficult to follow for a longer period of time the results of the study should not be diminished. The results might be of guidance both in clinical practice and for further studies. The optimal study for studying the value of nutritional surveillance would be to compare a study group with a control group. However, one might discuss the ethical dilemma to provide nutritional support to one group and not to another group of patients, considering that nutritional surveillance is seen as crucial during the treatment phase of patients with H and N cancer. Another limitation of our retrospective design is that it is not possible to perform a more thorough analysis of the effect of EN. It has generally been accepted that nutritional support and supplements are doing more good than harm in H and N cancer patients. Benefits from nutritional support, however, have been under debate in recent years. Moreover, one should keep in mind the theoretical risk that recurrence of malignancy and survival rate in the long run might be negatively affected by nutritional support. A study by Rabinovitch et al. [29] of 1,073 H and N cancer patients demonstrated poorer 5-year locoregional control and survival rate for patients that received nutritional support before treatment compared with patients who received nutritional support during treatment and patients who did not. However, the present findings do not lend support for a changed attitude toward the value of nutritional support in H and N cancer patients receiving RT or combined treatment. More studies on nutritional surveillance programmes with longer follow-up need to be carried out. Nutritional management of H and N cancer is still an important part of care to support patients during and after treatment, irrespective of less obvious effects on survival and infection prevention.
Conclusions
H and N cancer patients were found to lose weight after treatment, even though they received a nutritional surveillance programme of regular follow-ups up to 2 years after determination of treatment. The nadir of body weight was observed about 6 months after completion of RT. A significantly greater maximum weight loss was seen in patients undergoing combined modality treatment; a majority of them (68%) received EN, and it seemed as though this treatment could not prevent weight loss. The strongest prognostic predictor for maximum weight loss was tumour stage. Nutritional surveillance is important for all patients, especially in patients with a more severe disease stage or that need EN.
References
Vissink A, Jansma J, Spijkervet FKL, Burlage FR, Coppes RP (2003) Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 14:199–212. doi:10.1177/154411130301400305
Chasen MR, Bhargava R (2009) A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support Care Cancer 17:1345–1351. doi:10.1007/s00520-009-0684-5
Hayward MC, Shea AM (2009) Nutritional needs of patients with malignancies of the head and neck. Semin Oncol Nurs 25:203–211. doi:10.1016/j.soncn.2009.05.003
García-Peris P, Parón L, Velasco C et al (2007) Long-term prevalence of oropharyngeal dysphagia in head and neck cancer patients: impact on quality of life. Clin Nutr 26:710–717. doi:10.1016/j.clnu.2007.08.006
Batty J (2000) Nutrition. In: Feber T (ed) Head and neck oncology nursing. Whurr, London, pp 171–188
Capuano G, Grosso A, Gentile PC et al (2008) Influence of weight loss on outcomes in patients with head and neck cancer undergoing concomitant chemoradiotherapy. Head Neck 30:503–508. doi:10.1002/hed.20737
Bull DM (1975) Nutrition and tumor immunity: divergent effects of antitumor antibody. Cancer Res 35:3317–3319
De Luis DA, Izaola O, Cuellar L, Terroba MC, Martin T, Aller R (2009) High dose of arginine enhanced enteral nutrition in postsurgical head and neck cancer patients. A randomized clinical trial. Eur Rev Med Pharmacol Sci 13:279–283
Da De Luis, Izaola O, Aller R (2007) Nutritional status in head and neck cancer patients. Eur Rev Med Pharmacol Sci 11:239–243
Kubrak C, Olson K, Jha N et al (2010) Nutrition impact symptoms: key determinants of reduced dietary intake, weight loss, and reduced functional capacity of patients with head and neck cancer before treatment. Head Neck 32:290–300. doi:10.1002/hed.21174
Holder H (2003) Nursing management of nutrition in cancer and palliative care. Br J Nurs 12:667–668, 670, 672–674
Ouwens MM, Hermens RR, Hulscher MM et al (2009) Impact of an integrated care program for patients with head and neck cancer on the quality of care. Head Neck 31:902–910. doi:10.1002/hed.21041
Ehrsson YT, Hellström PM, Brismar K, Sharp L, Langius-Eklöf A, Laurell G (2010) Explorative study on the predictive value of systematic inflammatory and metabolic markers on weight loss in head and neck cancer patients undergoing radiotherapy. Support Care Cancer 18:1385–1391. doi:10.1007/s00520-009-0758-4
McRackan TR, Watkins JM, Herrin AE et al (2008) Effect of body mass index on chemoradiation outcomes in head and neck cancer. Laryngoscope 118:1180–1185. doi:10.1097/MLG.0b013e31816fca5c
Ehrsson YT, Langius-Eklöf A, Bark T, Laurell G (2004) Percutaneous endoscopic gastrostomy (PEG)—a long-term follow-up study in head and neck cancer patients. Clin Otolaryngol Allied Sci 29:740–746
Scolapio J, Spangler P, Romano M, McLaughlin M, Salassa J (2001) Prophylactic placement of gastrostomy feeding tubes before radiotherapy in patients with head and neck cancer: Is it worthwhile? J Clin Gastroenterol 33:215–217
National food administration in Sweden (2003) Mat och näring för sjuka inom vård och omsorg. 23
Isenring EA, Bauer JD, Capra S (2007) Nutrition support using the American Dietetic Association medical nutrition therapy protocol for radiation oncology patients improves dietary intake compared with standard practice. J Am Diet Assoc 107:404–412. doi:10.1016/j.jada.2006.12.007
Lee H, Havrila C, Bravo V et al (2008) Effect of oral nutritional supplementation on weight loss and percutaneous endoscopic gastrostomy tube rates in patients treated with radiotherapy for oropharyngeal carcinoma. Support Care Cancer 16:285–289. doi:10.1007/s00520-007-0313-0
Nugent B, Lewis S, O’Sullivan JM (2010) Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database Syst Rev, Issue 3. Article no.: CD007904
Ahlberg A, al-Abany M, Alevronta E et al (2010) Esophageal stricture after radiotherapy in head and neck cancer patients: experience of a single institution over 2 treatment periods. Head Neck 32:452–461. doi:10.1002/hed.21201
Corry J, Poon W, McPhee N et al (2009) Prospective study of percutaneous endoscopic gastrostomy tubes versus nasogastric tubes for enteral feeding in patients with head and neck cancer undergoing (chemo) radiation. Head Neck 31:867–876. doi:10.1002/hed.21044
Nguyena NP, Northb D, Smith HJ et al (2006) Safety and effectiveness of prophylactic gastrostomy tubes for head and neck cancer patients undergoing chemoradiation. Surg Oncol 15:199–203. doi:10.1016/j.suronc.2006.12.002
Ravasco P, Monteiro-Grillo I, Vidal PM, Camilo ME (2003) Nutritional deterioration in cancer: the role of disease and diet. Clin Oncol 15:443–450. doi:10.1016/S0936-6555(03)00155-9
Silver HJ, Dietrich MS, Murphy BA (2007) Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck 29:893–900. doi:10.1002/hed.20607
Nourissat A, Bairati I, Samson E et al (2010) Predictors of weight loss during radiotherapy in patients with stage I or II head and neck cancer. Cancer 116:2275–2283. doi:10.1002/cncr.25041
Pedruzzi PA, Kowalski LP, Nishimoto IN, Oliveira BV, Tironi F, Ramos GH (2008) Analysis of prognostic factors in patients with oropharyngeal squamous cell carcinoma treated with radiotherapy alone or in combination with systemic chemotherapy. Arch Otolaryngol Head Neck Surg 134:1196–1204
Isenring E, Cross G, Daniels L, Kellett E, Koczwara B (2006) Validity of the malnutrition screening tool as effective predictor of nutritional risk in oncology outpatients receiving chemotherapy. Support Care Cancer 14:1152–1156. doi:10.1007/s00520-006-0070-5
Rabinovitch R, Grant B, Berkey BA et al (2006) Impact of nutrition support on treatment outcome in patients with locally advanced head and neck squamous cell cancer treated with definitive radiotherapy: a secondary analysis of RTOG trial 90–03. Head Neck 28:287–296. doi:10.1002/hed.20335
Acknowledgements
The study was supported by grants from the Swedish Cancer Society, the Karolinska Institutet, the Swedish Nutritional Network for Nurses and the Swedish Laryngeal Cancer Foundation.
Disclosures
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ehrsson, Y.T., Langius-Eklöf, A. & Laurell, G. Nutritional surveillance and weight loss in head and neck cancer patients. Support Care Cancer 20, 757–765 (2012). https://doi.org/10.1007/s00520-011-1146-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-011-1146-4