Abstract
Introduction
Mucositis is a major complication in myeloablative therapy, which often necessitates advanced pharmacological pain treatment, including i.v. opioids. Attempts to prevent oral mucositis have included oral cryotherapy, which has been shown to reduce mucositis, but there is a lack of knowledge concerning the effect of oral cryotherapy on opioid use by reducing the mucositis for patients treated with myeloablative therapy before bone marrow transplantation (BMT).
Aim
The aim of the present study was to evaluate if oral cryotherapy could delay or alleviate the development of mucositis and thereby reduce the number of days with i.v. opioids among patients who receive myeloablative therapy before BMT.
Materials and methods
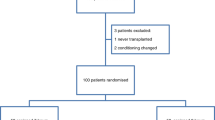
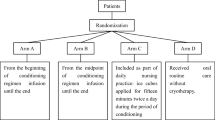
Eighty patients 18 years and older, scheduled for BMT, were included consecutively and randomised to oral cryotherapy or standard oral care. A stratified randomisation was used with regard to type of transplantation. Intensity of pain, severity of mucositis and use of opioids were recorded using pain visual analogue scale (VAS) scores, mucositis index scores and medical and nursing charts.
Results
This study showed that patients receiving oral cryotherapy had less pronounced mucositis and significantly fewer days with i.v. opioids than the control group. In the autologous setting, cryotherapy patients also needed significantly lower total dose of opioids.
Conclusion
Oral cryotherapy is an effective and well-tolerated therapy to alleviate mucositis and consequently reduce the number of days with i.v. opioids among patients treated with myeloablative therapy before BMT.


Similar content being viewed by others
References
McGuire D et al (1993) Pattern of mucositis and pain in patients receiving preparatic chemotherapy and bone marrow transplantation. Oncol Nurs Forum 20:1493–1502
Woo SB et al (1993) A longitudinal study of oral ulcerative mucositis in bone marrow transplant recipients. Cancer 72(5):1612–1617
Sonis S (1998) Mucositis as a biological process: a new hypothesis for the development of chemotherpy-induced stomatotoxity. Oral Oncol 34:39–43
Bellm LA et al (2000) Patient reports of complications of bone marrow transplantation. Support Care Cancer 8(1):33–39
Sonis ST et al (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 19(8):2201–2205
Sonis S (2004) The pathobiology of mucositis. Nat Rev Cancer 4:227–284
Lalla R, Wang Z, Sonis S (2005) Role of the cyclooxygenase pathway in chemotherapy-induced oral mucositis. In: 17th MASCC/ISOO international symposium. Geneva, Switzerland
Lunn R (1998) Oral management of the cancer patient. Part II: chemotherapy. Probe 32(2):58–65, 68
Sonis ST et al (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9 Suppl):1995–2025
Miaskowski C (1990) Oral complications of cancer therapies. Management of mucositis during therapy. NCI Monogr (9):95–98
Persson L, Hallberg I (1995) Acute leukaemia and malignant lymphoma patients’ experiences of disease, treatment and nursing care during the active treatment phase: an explorative study. Eur J Cancer Care 4(3):133–142
McGuire DB (2004) Occurrence of cancer pain. J Natl Cancer Inst Monogr (32):51–56
McGuire DB et al (1998) Acute oral pain and mucositis in bone marrow transplant and leukemia patients: data from a pilot study. Cancer Nurs 21(6):385–393
Meropol NJ et al (2003) Randomized phase I trial of recombinant human keratinocyte growth factor plus chemotherapy: potential role as mucosal protectant. J Clin Oncol 21(8):1452–1458
Larson L et al (2000) Comparison of perceived symptoms of patients undergoing bone marrow transplant and the nurses caring for them. Oncol Nurs Forum 20:81–87
Sonis S, Woods P, White B (1990) Oral complications of cancer therapies. Pretreatment oral assessment. Natl Cancer Inst Monogr 9:29–32
Zerbe MB et al (1992) Relationships between oral mucositis and treatment variables in bone marrow transplant patients. Cancer Nurs 15(3):196–205
Muus P et al (1992) Intencification of the conditioning regimen for allogeneic bone marrow transplantation in recipients of T-cell deplated grafts by the addition of anthracyclines. Leuk Lymphoma 7:11–14
Brincker H, Christensen BE (1993) Acute mucocutaneous toxicity following high-dose hydroxyurea. Cancer Chemother Pharmacol 32(6):496–497
Jones J et al (2006) Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer 14:505–515
Mahood DJ et al (1991) Inhibition of fluorouracil-induced stomatitis by oral cryotherapy. J Clin Oncol 9(3):449–452
Cascinu S et al (1994) Oral cooling (cryotherapy), an effective treatment for the prevention of 5-fluorouracil-induced stomatitis. Eur J Cancer B Oral Oncol 30B(4):234–236
Tartarone A et al (2005) Prevention of high-dose melphalan-induced mucositis by cryotherapy. Leuk Lymphoma 46:633–634
Yokomizo H et al (2004) Prophylactic efficacy of allopurinol ice ball for leucovorin/5 fluorouracil therapy-induced stomatitis. Anticancer Res 24:1131–1134
Aisa Y et al (2005) Oral Cryotherapy for the prevention of high-dose melphalan-induced stomatitis in allogeneic hematopoietic stem cell transplant recipients. Support Care Cancer 13(4):266–269
Mori T et al (2006) Brief oral cryotherapy for the prevention of high-dose melphalan-induced stomatitis in allogeneic hematopoietic stem cell transplant recipients. Support Care Cancer 14(4):392–395
Migliorati C, Oberle-Edwards L, Schubert M (2006) The role of alternative and natural agents, cryotherapy and/or laser for management of alimentary mucositis. Support Care Cancer 14:533–540
Karagözoglu S, Ulusoy M (2004) Chemotherapy: the effect of oral cryotherapy on the development of mucositis. Journal of Clinical Nursing 14:754–765
Lilleby K et al (2006) A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration or oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant 37:1031–1035
Worthington H, Clarkson J, Eden O (2006) Interventions for preventing oral mucositis for patients with cancer receiving treatment (review). Cochrane Database Syst Rev 2:CD000978
Rocke L et al (1993) A randomized clinical trial of two different duration of oral cryotherapy for prevention of 5-fluorouracil-related stomatitis. Cancer 72(7):2234–2238
Sonis ST et al (1999) Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer 85(10):2103–2113
Rubenstein EB et al (2004) Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100(9 Suppl):2026–2046
Blijlevens N, Donnelly J, DePauw B (2005) Inflammatory response to mucosal barrier injury after myeloablative therapy in allogeneic stem cell transplant recipients. Bone Marrow Transplant 36:703–707
Acknowledgements
This study was in part supported by FoU funds, Uppsala läns landsting, Sweden. The authors express their sincere appreciation to the nursing and physician staff of the participating centre.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Details of cytostatic treatment
In the study group, 15 patients in the EXP group and 13 patients in the CTR group received BEAC as conditioning regime [300 mg/m2 karmustin (Becenun®) on one occasion, 100 mg/m2 etoposid (Vepesid®) on eight occasions during 5 days, 100 mg/m2 cytarabin (Cytocar®) on eight occasions during 4 days and 35 mg/kg cyklofosfamid (Sendoxan®) on four occasions during 4 days]. Thirteen patients in the EXP group and 16 patients in the CTR group with myeloma received high-dose melphalan (Alkeran®) 200 mg/m2 on 1 single day. One patient in the CTR group and one patient in the EXP group received fludarabine (Fludara®) 30 mg/m2. Two patients in the EXP group and three patients in the CTR group treated for acute myelogenous leukaemia (AML) and one patient treated for myeloma received busulfan (Myleran®) at a dose of 1 mg/kg, four times/day during 4 days, and cyklofosfamid (Sendoxan®) at a dose of 60 mg/kg once a day during 2 days. Four patients in the EXP group and one patient in the CTR group were treated for acute lymphoblastic leukaemia (ALL). They received TBI (one fraction) at one occasion. Methotrexate, intrathecal injection was given at a dose of 10 mg/m2 (maximum, 15 mg) at one occasion. Furthermore, they received vincristine (Oncovin®) 1.5 mg/m2 on one occasion and daunorubicin (Cerubidin®) 30 mg/2 on one occasion. Cytarabin (Cytocar®) was given at a dose of 500 mg/m2 once a day during 5 days and teniposide (Vumon®) at a dose of 200 mg/m2 at one occasion. Cyklofosfamid (Sendoxan®) 40 mg/kg was administrated once daily for 2 days. One patient in the EXP group and one patient in the CTR group treated for testicular cancer received similar regimes. They consisted of etoposid (Vepesid®) 60 mg/m2 once a day for 4 days, carboplatin (Paraplatin®) given at a dose of 7× (GFR+25) mg once a day for 4 days and cyklofosfamid (Sendoxan®) 1,500 mg/m2 administrated once a day for 4 days. Two patients in the experimental group were treated for chronic myelocytic leukaemia (CML) with different regimes, regarding development of CML.
Rights and permissions
About this article
Cite this article
Svanberg, A., Birgegård, G. & Öhrn, K. Oral cryotherapy reduces mucositis and opioid use after myeloablative therapy—a randomized controlled trial. Support Care Cancer 15, 1155–1161 (2007). https://doi.org/10.1007/s00520-007-0245-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-007-0245-8