Abstract
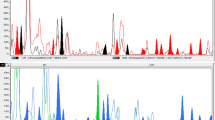
A male-specific amplified fragment length polymorphism (AFLP) marker was identified in the functionally dioecious fig species, Ficus fulva. A total of 89 polymorphic fragments from three primer combinations were produced, of which one (246 bp) was present in all males (n=23) and absent in all females (n=24) of two populations. This strong association suggests a tight chromosomal linkage between the AFLP marker and the sex-controlling locus. Further analysis indicated that the marker segregated in open-pollinated progenies from natural populations in a 1:1 ratio (n=156), implying that males are the heterogametic sex. Chromosome preparations showed no evidence for morphologically distinct sex chromosomes. The low frequencies of associated markers argue against a morphologically cryptic non-recombining sex chromosome. The sex-locus is therefore likely to be autosomal. The male-specific AFLP marker was sequenced and converted into a sequence characterised amplified region (SCAR) marker. This SCAR marker produced a fragment of equal size in males and females, suggesting that sequence divergence between male- and female-specific chromosomal regions is low.


Similar content being viewed by others
References
Alstrom-Rapaport C, Lascoux M, Yang YC, Roberts G, Tuskan GA (1998) Identification of a RAPD marker linked to sex determination in the basket willow (Salix viminalis L.). J Hered 89:44–49
Anstett MC, Hossaert-McKey M, Kjellberg F (1997) Figs and fig pollinators: evolutionary conflicts in a coevolved mutualism. Trends Ecol Evol 12:94–99
Arumuganathan K, Earle ED (1991) Nuclear DNA content of some important plant species. Plant Mol Biol Rep 9:211–215
Bawa KS (1980) Evolution of dioecy in flowering plants. Annu Rev Ecol Syst 11:15–39
Berg CC (1989) Classification and distribution of Ficus. Experientia 45:605–611
Charlesworth D (2001) Plant sex determination and sex chromosomes. Heredity 88:94–101
Compton SG, Ross SJ, Thornton IWB (1994) Pollinator limitation in fig tree reproduction on the island of Anak Krakatau (Indonesia). Biotropica 26:180–186
Condit IJ (1928) Cytological and morphological studies in the genus Ficus. I. Chromosome number and morphology in seven species. Univ Calif Publ Bot 11:233–244
Condit IJ (1934) Cytological and morphological studies in the genus Ficus. II. Chromosome number and morphology in thirty-one species. Univ Calif Publ Bot 17:61–74
Condit IJ (1964) Cytological studies in the genus Ficus III. Chromosome numbers in sixty-two species. Madroño 17:153–154
Conn JS, Blum U (1981) Sex ratio of Rumex hastulatus: the effect of environmental factors and certation. Evolution 35:1108–1116
Corner EH (1965) Check-list of Ficus in Asia and Australasia with keys to identification. Gard Bull Singapore 21:1–186
Costich DE, Meagher TR, Yurkow EJ (1991) A rapid means of sex determination in Silene latifolia by use of flow cytometry. Plant Mol Biol Rep 9:359–370
Deputy JC, Ming R, Ma H, Liu Z, Fitch MMM, Wang M, Manshardt R, Stiles JI (2002) Molecular markers for sex determination in papaya (Carica papaya L.). Theor Appl Genet 106:107–111
Docters van Leeuwen WM (1936) Krarakatu, 1883–1933 A. Ann Jard Botan Buitenzorg. Brill, Leiden
Grant SR (1999) Genetics of gender dimorphism in higher plants. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin Heidelberg New York, pp 247–274
Harrison RD, Yamamura N (2003) A few more hypotheses for the evolution of dioecy in figs (Ficus, Moraceae). Oikos 100:628–635
Hormaza JI, Dollo L, Polito VS (1994) Identification of a RAPD marker linked to sex determination in Pistacia vera using bulked segregant analysis. Theor Appl Genet 89:9–13
Janzen DH (1979) How to be a fig. Annu Rev Ecol Syst 10:13–51
Krause O (1930) Cytologische studien bei den Urticales. Dtsch Bot Ges Ber 48:9–13
Mandolino G, Carboni A, Forapani S, Faeti V, Ranalli P (1999) Identification of markers linked to the male sex in dioecious hemp (Cannabis sativa I.). Theor Appl Genet 98:86–92
Mulcahy DL, Weeden NF, Kesselli R, Carroll SB (1992) DNA probes for the Y-chromosome of Silene latifolia, a dioecious angiosperm. Sex Plant Reprod 5:86–88
Nordborg M, Tavar S (2002) Linkage disequilibrium: what history has to tell us. Trends Genet 18:83–90
Nordborg M, Borevitz JO, Bergelson J, Berry CC, Chory J, Hagenblad J, Kreitman M, Maloof JN, Noyes T, Oefner PJ, Stahl EA, Weigel D (2002) The extent of linkage disequilibrium in Arabidopsis thaliana. Nat Genet 30:190–193
Ohri D, Khoshoo TN (1987) Nuclear DNA contents in the genus Ficus (Moraceae). Plant Syst Evol 156:1–4
Pijnacker LP, Ferwerda MA (1984) Giemsa C-banding of potato chromosomes. Can J Genet Cytol 26:415–419
Purrington CB, Smith J (1995) Sexual dimorphism of dormancy and survivorship in buried seeds of Silene latifolia. J Ecol 83:795–800
Remington DL, Thornsberry JM, Matsuoka Y, Wilson LM, Whitt SR, Doebley J, Kresovich S, Goodman MM, Buckler ES IV (2001) Structure of linkage disequilibrium and phenotypic associations in the maize genome. Proc Natl Acad Sci USA 98:11479–11484
Rogstad SH (1992) Saturated NaCl-CTAB solution as a means of field preservation of leaves for DNA analyses. Taxon 41:701–708
Ruas CF, Fairbanks DJ, Evans RF, Stutz HC, Andersen WR, Ruas PM (1998) Male-Specific DNA in the dioecious species Atriplex garrettii (Chenopodiaceae). Am J Bot 85:162–167
Sakai AK, Weller SG (1999) Gender and sexual dimorphism in flowering plants: a review of terminology, biogeographic patterns, ecological correlates, and phylogenetic approaches. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, Berlin Heidelberg New York, pp 1–31
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Storey WB (1955) Sex inheritance in figs. California Fig Institute. Proceedings of the Annual Research Conference 9:15–17
Storey WB (1975) Figs. In: Janick J, Moore JN (eds) Advances in fruit breeding. EAGR vol 38 number 12. Purdue University Press, West Lafayette, Ind., pp 568–589
Tas ICQ, van Dijk PJ (1999) Crosses between sexual and apomictic dandelions (Taraxacum). I. The inheritance of apomixis. Heredity 83:707–714
Valdeyron G, Lloyd DG (1979) Sex differences and flowering phenology in the common fig, Ficus carica L. Evolution 33:673–685
Vos P, Hogers R, Bleeker M, Reijans M, van der Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Weiblen GD (2000) Phylogenetic relationships of functionally dioecious Ficus (Moraceae) based on ribosomal DNA sequences and morphology. Am J Bot 97:1342–1357
Yampolsky C, Yampolsky H (1922) Distribution of the sex forms in the phanerogamic flora. Bibl Genet 3:1–62
Zang YH, Distilio VS, Rehman F, Avery A, Mulcahy D, Kesseli R (1998) Y chromosome specific markers and the evolution of dioecy in the genus Silene. Genome 41:141–147
Acknowledgements
We acknowledge Hans de Jong and Henny Verhaar (University of Wageningen, the Netherlands) for the cytogenetic analyses, Piet Stam (University of Wageningen, the Netherlands) for statistical advice and Sophie Ahmed (University of Leeds, UK) for sequencing the male-specific AFLP fragment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publication 3311 NIOO-KNAW Netherlands Institute of Ecology
Rights and permissions
About this article
Cite this article
Parrish, T.L., Koelewijn, H.P. & van Dijk, P.J. Identification of a male-specific AFLP marker in a functionally dioecious fig, Ficus fulva Reinw. ex Bl. (Moraceae). Sex Plant Reprod 17, 17–22 (2004). https://doi.org/10.1007/s00497-004-0208-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-004-0208-x