Abstract
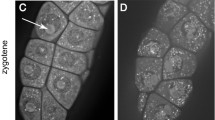
A meiotic time-course for Arabidopsis pollen mother cells has been established based on BrdU pulse-labelling of nuclear DNA in the meiotic S-phase. Labelled flower buds were sampled at intervals and the progress of labelled cells through meiosis assessed by anti-BrdU antibody detection. The overall duration of meiosis from the end of meiotic S-phase to the tetrad stage, at 18.5°C, was 33 h, which is about three times longer than the mitotic cell cycle in seedlings. The onset of leptotene was defined by reference to the loading of the axis-associated protein Asy1, and this permitted the detection of a definite G2 stage, having a maximum duration of 9 h. It is likely, from two independent sources of evidence, that the meiotic S-phase has a duration similar to that of G2. The durations of leptotene and zygotene/pachytene are 6 h and 15.3 h, respectively, but the remaining meiotic division stages are completed very rapidly, within 3 h. The establishment of a meiotic time-course provides a framework for determining the relative timing and durations of key molecular events of meiosis in Arabidopsis in relation to cytologically defined landmarks. In addition, it will be important in a broader developmental context for determining the timing of epigenetic mechanisms that are known or suspected to occur during meiosis.



Similar content being viewed by others
References
Albini SM (1994) A karyotype of the Arabidopsis thaliana genome derived from synaptonemal complex analysis at prophase I of meiosis. Plant J 5:665–672
Armstrong SJ, Jones GH (2003) Meiotic cytology and chromosome behaviour in wild-type Arabidopsis thaliana. J Exp Bot 54:1–10
Armstrong SJ, Franklin FCH, Jones GH (2001) Nucleolus-associated telomere clustering and pairing precede meiotic chromosome synapsis in Arabidopsis thaliana. J Cell Sci 114:4207–4217
Armstrong SJ, Caryl AP, Jones GH, Franklin FCH (2002) Asy1, a protein required for meiotic chromosome synapsis, localizes to axis-associated chromatin in Arabidopsis and Brasssica. J Cell Sci 115:3645–3655
Bennett MD (1971) The duration of meiosis. Proc R Soc London Ser B 178:277–299
Bennett MD, Leitch IJ (1995) Nuclear DNA amounts in Angiosperms. Ann Bot 76:113–176
Bennett MD, Chapman V, Riley R (1971) The duration of meiosis in pollen mother cells of wheat, rye and Triticale. Proc R Soc London Ser B 178:259–275
Bennett MD, Cox AV, Leitch IJ (2000) Angiosperm DNA C-values database. http://www.rbgkew.org.uk/cval/database1.html
Bhatt AM, Canales C, Dickinson HG (2001) Plant meiosis: the means to 1n. Trends Plant Sci 6:114–120
Callan HG (1972) Replication of DNA in the chromosomes of eukaryotes. Proc R Soc London Ser B 181:19–41
Callan HG, Perry PE (1977) Recombination in male and female meiocytes contrasted. Philos Trans R Soc London Ser B 277:227–233
Callan HG, Taylor JH (1968) An autoradiographic study of the time course of male meiosis in the newt Triturus vulgaris. J Cell Sci 3:615–626
Caryl AP, Armstrong SJ, Jones GH, Franklin FCH (2000) A homologue of the yeast HOP1 gene is inactivated in the Arabidopsis meiotic mutant asy1. Chromosoma 109:62–71
Caryl AP, Jones GH, Franklin FCH (2003) Dissecting plant meiosis using Arabidopsis thaliana mutants. J Exp Bot 54:25–38
Chandley AC, Bateman AJ (1962) Timing of spermatogenesis in Drosophila melanogaster using tritiated thymidine. Nature 193:299–300
Crone M, Levy E, Peters H (1965) The duration of premeiotic DNA synthesis in mouse. Exp Cell Res 39:678–688
Erickson RO (1948) Cytological and growth correlation in the flower bud and anther development of Lilium longiflorum. Am J Bot 35:729–739
Goodrich J, Tweedie S (2002) Remembrance of things past: chromatin remodelling in plant development. Annu Rev Cell Dev Biol 18:707–746
Heller CG, Clermont Y (1963) Spermatogenesis in man: an estimate of its duration. Science 140:184–186
Holm PB (1977) The premeiotic DNA replication of euchromatin and heterochromatin in Lilium longiflorum (Thunb.). Carlsberg Res Commun 42:249–281
Hunter N, Kleckner N (2001) The single-end invasion: an asymmetric intermediate at the double-strand break to double-Holliday junction transition of meiotic recombination. Cell 109:59–70
Ito M, Stern H (1967) Studies of meiosis in vitro. I. In vitro culture of meiotic cells. Dev Biol 16:36–53
Kofman-Alfaro S, Chandley AC (1970) Meiosis in the male mouse. An autoradiographic investigation. Chromosoma 31:404–420
Latos-Bielenska A, Vogel W (1990) Frequency and distribution of chiasmata in Syrian hamster spermatocytes studied by the BrdU antibody technique. Chromosoma 99:267–272
Li E (2002) Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 3:662–673
Lima-de-Faria A (1950) Meiosis and pollen mitosis in rye under controlled conditions. Hereditas 36:106–109
Lima-de Faria A (1965) Labelling of the cytoplasm and the meiotic chromosomes of Agapanthus with H3-thymidine. Hereditas 53:1–18
Mercier R, Vezon D, Bullier E, Motamayor JC, Sellier A, Lefevre F, Pelletier G, Horlow C (2001) Switch1 (Swi1): a novel protein required for the establishment of sister chromatid cohesion and for bivalent formation at meiosis. Genes Dev 15:1859–1871
Mercier R, Armstrong SJ, Horlow C, Jackson NP, Makaroff CA, Vezon D, Pelletier G, Jones GH, Franklin FCH (2003) The meiotic protein Swi1 is required for axial element formation and recombination initiation in Arabidopsis thaliana. Development 130:3309–3318
Naysmyth K (2001) Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu Rev Genet 35:673–745
Oakberg EF (1956) Duration of the spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat 99:9998–1002
Padmore R, Cao L, Kleckner N (1991) Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell 66:1239–1256
Taylor JH (1950) The duration of differentiation in excised anthers. Am J Bot 31:137–143
Van't Hof J (1988) Chromosomal replicons of higher plants. In: Gustafson JP, Appels R (eds) Chromosome structure and function. 18th Stadler genetics symposium. Plenum, New York
Van't Hof J, Kuniyuki A, Bjerknes CA (1978) The size and number of replicon families of chromosomal DNA of Arabidopsis thaliana. Chromosoma 68:269–285
Williamson DH, Johnston LH, Fennell DJ, Simchen G (1983) The timing of the S phase and other nuclear events in yeast meiosis. Exp Cell Res 145:209–217
Zickler D, Kleckner N (1998) The leptotene-zygotene transition of meiosis. Annu Rev Genet 32:619–697
Zickler D, Kleckner N (1999) Meiotic chromosomes: integrating structure and function. Annu Rev Genet 33:603–704
Acknowledgement
We acknowledge the financial support provided by the Biotechnology and Biological Sciences Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armstrong, S.J., Franklin, F.C.H. & Jones, G.H. A meiotic time-course for Arabidopsis thaliana . Sex Plant Reprod 16, 141–149 (2003). https://doi.org/10.1007/s00497-003-0186-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-003-0186-4