Abstract
Key message
Black pine and Maritime pine exhibit considerable differences in growth phenology across elevation belts with a 1-month delay for xylogenesis and increasing growth rates from low, mid to high elevations.
Abstract
Investigating seasonal wood formation is crucial to understand tree growth responses to climate impact. The present study quantifies the variability of xylogenesis along an elevation gradient on the Mediterranean island of Corsica, where two native pine species (Pinus nigra Arnold ssp. laricio Maire and Pinus pinaster Aiton) grow from the upper tree line to sea level, partly in sympatry. We extracted microcores from 35 trees at 5 sites along an East–West transect from the coasts (10 m asl) to the island's central mountain ridge (1600 m asl) during bi-weekly sampling campaigns between 2017 and 2019. We applied generalised additive models to detect radial growth differences in elevation and species along with minimum temperatures for growth initiation. We found that trees in low elevations experienced over 2 months longer growth periods with higher maximum growth rates than in high elevations. The results show a 1-month delay for the beginning of tracheid formation between the low, mid and high-elevation belts but comparable timing for its termination. At the sites where both species co-occur, P. nigra accumulates more radial cells in a shorter time than P. pinaster. Trees at the two contrasting coastal sites have similar growth period lengths, but exhibit a time shift of over a month. Minimum temperatures around growth initiation vary between 3.1 °C for P. nigra and 9.9 °C for P. pinaster. These findings emphasise the high plasticity in the growth behaviour of one of the most widespread tree genera in Mediterranean and temperate zones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tracing intraannual wood formation, a process called xylogenesis, is paramount to better understand how tree growth responds to climatic influences. Trees experience secondary growth (i.e. girth increase of branches, stems and roots) to varying extents throughout their lifecycle. After creation through cell division in the vascular cambium, the xylem cells oriented towards the pith undergo three phases of differentiation (Wilson et al. 1966). In the initial phase of radial cell enlargement, the unlignified cell walls are composed of cellulose and hemicellulose. After cell enlargement is terminated, the secondary cell walls are thickened and lignin is incorporated between each cell's primary and secondary walls. This cell-wall thickening phase is of particular importance for trees, because it enhances the tissue's stability and enables trees to become large individuals. Lastly, programmed cell death occurs. During this relatively quick process, the cell lumen is cleared of its contents, and the water-conducting xylem cells become fully functional. The cells are then considered mature. The critical dates of each phase's onset, cessation and duration are crucial for the organism's development (Rathgeber et al. 2016). The differentiation of the xylem cells and the occurrence of the different xylogenetic phases, however, is a very plastic process that is influenced by the environmental conditions during cell formation and differentiation. Therefore, precise knowledge about the impact of climatic factors on xylogenesis can be helpful to develop growth models under expected future climatic conditions (e.g. Delpierre et al. 2019). A recent meta-study considering cambial phenology over the Northern Hemisphere has underlined that climatic warming led to longer growing seasons in boreal, temperate and Mediterranean forests. However, semi-arid and dry subalpine forests do not benefit due to water loss and/or more frequent early spring frosts (Gao et al. 2022). Another study, investigating spring phenology of Northern Hemisphere conifers, identified a mean annual temperature threshold of 4.9 ± 1.1 °C, above which the response of xylem phenology to rising temperatures significantly declines (Huang et al. 2023).
Various methods to generate intraannual growth models based on categorised cell counts have been published. Gompertz functions (Rossi et al. 2003) have been used extensively, but are not always appropriate due to the rigidness of the sigmoid shape. In the past decade, generalised additive models (GAM) (Hastie and Tibshirani 1986; Cuny et al. 2013; Cocozza et al. 2016) have been introduced, and recently Bayesian regularisation algorithms (BRNN) have been proposed (Jevšenak et al. 2022) as an instrument to analyse xylogenesis changes throughout the growing period and to identify the triggering environmental factors.
Since the introduction of tools specifically designed to collect microcores from the cambial and xylem differentiation zone, like e.g. the Trephor microcorer (Rossi et al. 2006a; b), studies of tissue formation using microcores have been standardised, resulting in an improved insight into the process of xylogenesis. During the past decade, xylogenesis has been studied in various biomes, e.g. in the tropics (Krepkowski et al. 2011; Pumijumnong et al. 2021), in temperate environments (Gričar et al. 2014) and in subalpine tree-line ecotones (Gruber et al. 2010; Ren et al. 2018). Another region of interest is the Mediterranean, with its typically warm and dry summers and cool and humid winters. Growth periods in the Mediterranean region are longer than in temperate regions, but trees typically show lower growth rates (Rathgeber 2019). In climate regimes showing a pronounced dry period in summer and a wet autumn, wood formation can be interrupted which leads to a bimodal growth pattern. However, how exactly this Mediterranean growth pattern is triggered by climate factors is not yet fully understood. In a water manipulation experiment, Vieira et al. (2020) found that additional irrigation in September did not result in noticeable differences in xylogenesis in Maritime pine (P. pinaster) in Portugal. Moreover, it is not yet known at which elevation the climatic conditions triggering a bimodal growth pattern are not met anymore. Furthermore, it is not clear if the same climate pattern triggers the same xylogenetic phases in different species.
The Mediterranean island of Corsica, France, is well suited to study tree growth along a broad elevation range. Corsican Black pine (Pinus nigra Arnold ssp. laricio Maire var. corsicana Loudon) and Maritime pine (Pinus pinaster Aiton) naturally occur along different elevation belts spanning from sea level to the upper treeline. Recent warming has already impacted the maximum latewood density of high-elevation Black pines on Corsica (Römer et al. 2021). Since Mediterranean climate is predicted to become warmer and more arid in the future (IPCC 2022) and Corsican pine populations are especially sensitive to drought (Versace et al. 2022), it remains a central question for tree physiology and tree-ring research, how exactly intraannual growth dynamics vary within a genus as a function of climate within a region. On the mountainous island of Corsica, pronounced climatic gradients from Mediterranean to temperate climate regimes occur on short horizontal distances along steep elevation gradients, providing a suitable setting to study the climatic impact on xylogenesis. Insights about growth responses of Corsican pines to climate variability have been gained on interannual (Häusser et al. 2019) and intraannual scales (Häusser et al. 2021), but not yet on a wood-anatomical basis. With several years of data, it is possible to provide a sufficient database to model radial growth with high accuracy on regional scales. Another important open question is why and when cold-induced winter and drought-induced summer cambial dormancy occurs along the elevation gradient. One temperature threshold often used to initiate cambial activity after winter dormancy in temperate to alpine regions is + 5 °C over several consecutive days (Gruber et al. 2009; Etzold et al. 2022). The coastal pines on Corsica form tree rings which indicates the regular occurrence of a dormant season (Häusser et al. 2019), although winter temperatures often do not drop below this threshold. Hence, it is not fully understood what the climatic limiting factors for cambial activation in the Mediterranean lowlands are and when exactly cambial inactivity occurs.
This study investigates the relationship between xylogenesis and climatic factors. We analysed the intraannual growth dynamics of P. nigra and P. pinaster over 3 consecutive years at five sites across an altitudinal gradient on the island of Corsica. With this study design, we were able to investigate growth dynamics and their forcing factors under Mediterranean, temperate and subalpine climatic conditions within one tree genus.
We hypothesised that: (1) the growth periods of Corsican pine trees are longer in lower elevation belts than in higher elevation belts, due to a longer thermal growing season. (2) The maximum growth rates are lower in lower elevation belts than in higher elevations. (3) The minimum temperature for growth initiation after cambial dormancy at higher elevations is 5 °C, and coastal pines are dormant in winter.
Through regular microcore sampling, subsequent thin sectioning, application of GAMs and on-site weather measurements, we aimed (1) to quantify the variation of xylogenesis in Corsican pines along the elevation gradient and (2) to reveal possible species-specific differences in xylogenesis between the two species. Furthermore, we attempted (3) to determine the minimum temperatures for growth initiation.
Methods
Study area, species and study design
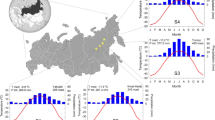
Corsica is an island in the western Mediterranean Sea with a north to south extension of 180 km and an east to west extension of 80 km. With its central mountain massif of elevations of up to 2700 m asl, it shows pronounced elevation gradients towards the east and west. The local climate on the island is diverse, depending on elevation and topography. The low-elevation belt experiences a characteristic Mediterranean climate with precipitation sums between 600 mm on the west and 800 mm on the east coast, with a precipitation peak in autumn and winter and a pronounced summer drought lasting from the beginning of June to the end of August. Annual mean temperatures are 15–16 °C, whilst annual monthly minimum temperatures are around 10 °C in January and February (MétéoFrance 2020a). With increasing elevation, the climatic regime gradually becomes more temperate and the drought phase, during the summer months, diminishes. The highest available meteorological station at 1030 m asl (Evisa, c.f. Fig. 1) records an annual mean temperature of 10.3 °C and a precipitation sum of 1438 mm (average for the period 1981–2010 (MétéoFrance 2020a). The upper tree-line runs in approximately 1800 m asl, where winter temperatures frequently drop below freezing and snow cover can persist over weeks, sometimes well into the beginning of spring.
Climate diagrams representing the high, middle and low-elevation belts. Daily data derived from the nearest 0.1° × 0.1° grid point of the dataset E-OBS (Cornes et al. 2018) from 1991 to 2020. Diagrams are produced with the R-package 'climatol' (Guijarro 2018). The table informs about the site features, pine species and sample size with biometric details about investigated trees. Site ID's refer to the respective cardinal direction (W = west and E = east) and elevation belt (H = high, M = medium, and L = low). σ represents standard deviation. Map shows the locations of the study sites (coloured circles). The schematic island profile illustrates the studied east–west transect, including the elevation ranges of the two pine species (PN = Pinus nigra ssp. laricio, PP = Pinus pinaster) with the mixed zone of range overlap between 750 and 1250 m asl
The investigated species are Corsican Black pine (Pinus nigra Arnold ssp. laricio Maire) and Maritime pine (Pinus pinaster Aiton). Both are native to Corsica and occur in two overlapping elevation belts. Pinus nigra (PN) grows from around 750 m asl to tree line and P. pinaster (PP) from sea level to around 1250 m asl. We monitored 5 trees per species at each site, adding up to 35 studied trees. For this study, we selected mostly relatively young, but not juvenile dominant trees with similar diameters at breast height (DBH) ranging from 35 to 45 cm (Fig. 1). Increment core samples of all trees were taken and tree-ring widths were measured (see Fig. S1 for raw data).
We selected five study sites and named them according to the cardinal direction of the slope of the mountain range (E = east and W = west) and elevation: below 750 m asl (L = low), where only P. pinaster is found, the mixed zone where both pine species co-occur between 750 and 1250 m asl (M = mid), and above 1250 m asl (H = high), where only P. nigra occurs. The highest site is considered subalpine due to its close proximity to the tree line. The soils at all sites are acidic due to the predominance of granites and other metamorphic rocks. However, water holding capacity as a consequence of soil depth varies locally. The deepest soils were found at the two coastal sites. The eastern site (EL), located on an extensive alluvial fan, exhibits a deep podzol, whereas we found a well-developed cambisol at the western site (WL). Cambisols were also found at the mid-elevation sites. The soil at the eastern site (EM) is deeper than the one in the west (WM). The soil at the highest site (EH) is a shallow podzolic cambisol close to a crest position of a mountain range.
Microscopic analyses and GAM fitting
We collected microcores from 35 trees at about 1–1.5 m stem height with an increment corer (Trephor; Costruzioni Meccaniche Carabin Carlo) to determine the time that each xylem cell spent in the different phases of enlargement and wall thickening (Rossi et al. 2006b). The sampling spots on the stems were separated by 5–10 cm to avoid wound effects. Sampling was conducted in total over 33 months with 2 years in bi-weekly intervals (April 2017–April 2019) and a third vegetation period in monthly intervals (May 2019–December 2019). Coastal sites were sampled year-round whereas this was not the case at higher elevation sites due to expected growth stop during the winter. Forty-two samples (2.4%) had to be rejected because of mechanical damages, resulting in a total number of one thousand seven hundred and twenty-two samples suitable for analysis.
After extraction from the stems, the samples were placed directly in microtubes filled with a 50% ethanol solution for transport and storage. In the laboratory, the samples were dehydrated with successive immersions in ethanol and limonene (ROTI Histol; Carl Roth) and then embedded in paraffin blocks (ROTI Plast; Carl Roth). We cut transverse sections of 8–12 µm thickness from the embedded microcores with a rotary microtome (RM2245; Leica Biosystems), stained them with safranin and astra blue, and permanently mounted them on glass slides for microscopic analyses and digital imaging (Smartzoom 5; Carl Zeiss) (Rossi et al. 2006a).
For each sample, we counted the number of cells (n) in the radial enlargement phase (ENL), cell-wall thickening phase (CWT) and mature cells (MAT) along five radial cell rows and computed means for the respective categories. Raw radial file data were checked for consistency and standardised with the previous year's cell count (Rossi et al. 2006a) with the 'CAVIAR' package (Rathgeber et al. 2018).
We applied generalised additive models (GAM) to detect how cambial activity changed across the island's different elevation belts, general aspects and pine species. GAMs were calculated for each tree for ENL, CWT and MAT phases based on cell count data from all 3 years (for single tree GAM statistics, see Table S1 and Fig. S2 for model fit). GAM provides an improved and more suitable approach than Gompertz functions for modelling intraannual wood formation (Cuny et al. 2013). Gompertz functions are asymptotic functions, normally used for monotonically increasing growth trends like the accumulation of MAT. Due to its ability to model non-linear fluctuations (Hastie and Tibshirani 1986), GAM can model cell formation in the different development stages of xylogenesis: ENL, CWT and MAT. We applied the 'gam' function of the 'mgcv' R package (Wood 2011) to create the models of the numbers of cells in different phases (nENL, nCWT, nMAT) of each tree using the quasi-Poisson distribution family. As shown by dendrometer data, there was a significant difference in radial tree growth along elevation and aspect between the years 2017 and 2018 (Häusser et al. 2021). To remove the effect of contrasting climate conditions, remedy the effect of missing data in the first 3 months of 2017 and enhance model performance, we focussed on fitting cell numbers for each tree over the entire study period (2017–2019), resulting in single modelled growth trends for each individual tree. The model diagnostics were assessed with the R package 'gratia' (Simpson 2022). Site means of the modelled cell numbers (nENL, nCWT, nMAT) and the 95% confidence interval for each site were calculated. All statistical analyses were performed using R software (R Core Team 2022) and RStudio (RStudio Team 2021).
From each cell differentiation phase, we determined its beginning (b) and cessation (c) of the respective day of the year (DOY), and calculated its duration (d) in days as the difference of (c) and (b). We used nENL to define the growing season length at each study site. The beginning (bENL) and cessation (cENL) of cambial growth (bENL) is assumed on the day of the year (DOY) when nENL surpasses and drops below the threshold of one cell. In the same way, we determined onset (bCWT), termination (cCWT) and duration (dCWT) of the cell-wall thickening phase and the onset of the mature phase (bMAT). Furthermore, we calculated daily growth rates (rENL) of the modelled numbers of enlarging cells to derive differences in growth changes between sites.
Recording and processing of meteorological data
We installed automated weather stations (Campbell Sci stations at EH, EM, and WM; Metek at EL and WL) with a mean horizontal distance of 760 m and a mean vertical distance of 50 m to our tree sites. Temperature, precipitation and relative humidity were recorded in high resolution (up to every minute) from May 2017 to November 2019. These data were then resampled into a daily format, and vapour pressure deficit (VPD) was calculated using mean temperature in the Magnus formula for saturation vapour pressure and relative humidity for vapour pressure (WMO 2018). To fill the time gap between the collection of tree samples and the establishment of the weather stations at the beginning of 2017 and other minor gaps in 2019, we used data from nearby stations of the French meteorological service MétéoFrance (MétéoFrance 2020a).
Three-year averages of daily precipitation, temperature and VPD values were calculated to investigate how average cambial growth is influenced by meteorological parameters. This approach was justified because the climatic conditions during the 3 study years were dry, wet and average, and all 3 years were equally warm compared to the long-term mean of the MétéoFrance stations.
Determination of climate–growth relationships
We performed series of correlation analyses using daily precipitation, temperature and vapour pressure deficit to analyse the relationships between the modelled number of cells and climate variables, averaged over 3 years, respectively. Preliminary statistical tests showed that the data were not normally distributed. Hence, we correlated modelled data with the daily climate parameters to determine the stem growth response to the three climatic variables with Kendall's rank correlation coefficient. Climate data were pooled over 21-day windows with a one-day shift and then correlated with site averages of nENL and nCWT. Additionally, relations between weather conditions and modelled beginnings and cessations of enlarging cell phases were established. For this calculation, we summarised precipitation, averaged temperature and VPD values from 10 days before to 5 days after bENL and cENL, respectively.
Results
Modelled cell phases along the elevation gradient
Tissue formation and cell differentiation clearly follow the elevation gradient. Trees in the low-elevation belt exhibit the onset and maximum of meristematic activity considerably earlier than the trees at the mid-elevation belt and especially the ones at the highest site (Fig. 2). The difference in beginning of ENL is shifted by at least 1 month between each elevation belt. Interestingly, this gradient is not observed during growth cessation. Trees at all sites except the coastal EL stopped forming new cells at approximately the same time around mid-September. Hence, the deduced growing season lengths along the elevation gradient vary considerably: coastal sites have an over 50 days longer growing period than mid-elevation sites, and these have a 30–40 days longer growing period compared to the high-elevation site (Table 1). bCWT shows a similar gradient, with an offset of at least 1 month between belts of increasing elevation. However, that is not the case for bMAT. Here, the delay between low- and mid-elevation sites is around 60 days, whereas the difference between the mid-elevation belt and the high site is less than 20 days. All nCWT curves of the high and mid-elevation sites experience retardation in summer between the initial development and their maxima from mid-September to early October. Only at high-elevation site EH, CWT is completed at the end of the year. At the mid-elevation sites, cessation of CWT occurs at the beginning of the following year (Table 1).
Modelled cell numbers in the enlarging (nENL), cell-wall thickening (nCWT) and mature (nMAT) phases of sampled trees along the elevation gradient. X-axes represent months and DOY. Coloured lines indicate fitted values obtained by generalised additive models, and shaded coloured areas indicate the corresponding 95% confidence intervals. If the shaded areas do not overlap, differences between sites within the same elevation belt are considered significant. Xylem phenology of P. nigra and P. pinaster is pooled at medium elevation sites (EM and WM), whilst at low (WL and EL) and highest (EH) elevation sites, xylem phenology represent exclusively PP and PN, respectively. Dashed vertical lines mark the timing of the highest number of cells for ENL, and the dotted horizontal line represents the one-cell threshold
It is worth to note the much larger difference between eastern and western slopes in the low-elevation sites compared to the mid-elevation sites. The tree growth seasonality of the low-elevation sites is shifted compared to the mid-elevation sites, where both pine species coexist (cfr. Fig. 2G with D). Whilst cell enlargement in the west (WL) already starts in December of the previous year, it is delayed to the beginning of February in the east (EL) (Fig. 2G). However, growth at both low-elevation sites peaks in late April. That leads to the situation where nENL at the coastal sites peaks in the same month in which cambial growth is just beginning in the high-elevation belt. The highest modelled ENL cell numbers occur at WL (8.1) and EM (7.9), and the lowest values at EH (6.0) (Fig. 2A, D, G). On the other hand, EL site has a strong tendency for bimodality by cambial resurgence from August to mid-November (from DOY 225 and 300 of Fig. 2G) after a temporary drop in nENL. Cell-wall thickening at the coastal sites differs substantially from that at higher elevations. However, the two coastal sites deviate strongly from each other: at site WL, the trees have an almost constant number of cells in the CWT phase throughout the year, whereas at the eastern coastal site EL, the number of cells in CWT phase strongly increases towards the end of the year (Fig. 2H). In general, trees at the eastern sites (EM, EL) accumulate more mature cells over a year than at western sites (WM, WL) (Fig. 2F, I), irrespective of altitude.
The growth rates (rENL) show that the coastal sites (WL and EL) experience the highest rates around the spring equinox, whilst the highest site EH reached this benchmark more than 2 months later (Fig. 3). Eastern sites reach the highest rate earlier than western sites, and the maximum values decrease with increasing elevation, with the prominent exception of P. nigra at EM. The highest site EH experiences a sharp secondary deceleration of nENL between DOY 200 and 225, indicating an abrupt slowing of cambial activity. The bimodality of growth at EL between mid-August and late October (DOY 230–295) is remarkably slow compared to the initial growth rate between January and April (Fig. 3).
Comparison of P. nigra and P. pinaster at co-occurring sites
Pinus pinaster starts and ends xylem growth earlier than P. nigra at sites WM and EM, where both species co-occur (Fig. 4). Hence, the growing period of P. nigra is shifted slightly forward, with higher cell counts in all phases, and maxima occur slightly later than in P. pinaster. P. nigra also halts growth later in the year, and hence has higher nMAT at both sites. The higher number of enlarging cells of P. nigra at WM site from late July to the end of September leads to a significant difference in nCWT and nMAT, when compared with P. pinaster. At EM site, the maximum number of nENL occurs simultaneously in both species, whereas it occurs half a month earlier for P. pinaster at WM. The eastern site (EM) has a higher maximum nENL, a later cENL and a higher final nMAT in both species. Maximum growth rates for P. pinaster occur later and are higher than for P. nigra at both sites (Fig. 3). Furthermore, growth rates of P. pinaster are also higher than those of P. nigra after the drought period, in autumn.
Generalised additive model (GAM) fittings for cell numbers in enlarging (ENL), wall thickening (CWT) and mature (MAT) phases at the eastern (A–C) and western (D–F) mid-elevation sites (EM and WM), where both studied pine species grow in sympatry. X-axes represent months and DOY. Coloured lines indicate fitted values obtained by GAMs, and shaded coloured areas indicate the corresponding 95% confidence intervals. If the shaded areas do not overlap, differences between sites are considered significant. Dashed vertical lines in nENL mark the timing of the highest number of cells, and the dotted horizontal line represents the one-cell threshold
Meteorological conditions from 2017 to 2019
The mean annual temperatures of each of the 3 analysed years were above the long-term mean for the whole island from 1981 to 2010 (MétéoFrance 2020b). The annual precipitation sum, on the other hand, varied substantially. The dry year 2017 (964 mm) was followed by a wet year in 2018 (1628 mm). The year 2019 was almost exactly in between (1214 mm). The 3-year mean values of the recorded temperature and precipitations clearly show the influence of the elevation gradient (Fig. 5). The highest daily precipitation sums and lowest temperatures occurred at the highest site (EH) (Fig. 5A). Although the eastern (EL) and the western coastal sites (WL) are both located close to sea level (10 and 15 m asl), their temperatures deviate in spring, summer and autumn, with consistently higher temperatures at WL.
The mean weekly precipitation sums decline with decreasing elevation (Fig. 5B). The difference between annual precipitation sums of the two mid-elevation (EM: 1325 mm and WM: 1574 mm) sites is slightly higher than between the two low-elevation sites (EL: 714 mm and WL: 561 mm). Regarding the dry summer period, the western sites suffer more from drought stress than the eastern sites. Mean VPD values during summer (JJA) are highest at WL (1.22 kPa) and WM (1.21 kPa) and lowest at EH (0.82 kPa). However, they are surprisingly low at EL (0.81 kPa).
Climate–growth relationship
The running correlations between climate parameters and the modelled nENL curves vary according to the climatic variable considered (Fig. 6). Significant correlations with precipitation are infrequent at all sites. Contrarily, VPD and especially temperature show much stronger relationships to nENL over extended periods. The correlations are highest and most continuous with temperature from the beginning of May to the end of June (c. DOY 120–180) and in autumn. There is a time frame of continuous high negative correlations that is getting smaller with increasing elevation. The highest site (EH) shows mostly significant positive correlations with temperature throughout the year, except in July. The pattern across the sites is also apparent with correlations of temperature and nCWT. However, it is shifted by 1 month, especially at the non-coastal sites. The VPD correlation patterns are less distinct, although they roughly resemble the patterns of temperature correlations in the higher elevations.
21-day running Kendall’s tau correlations with a 1-day shift and 20-day overlap of meteorological parameters and modelled cell numbers. Depicted are only significant correlations (p = 0.05) between modelled curves of nENL (upper panels), nCWT (lower panels), and precipitation, temperature and VPD. Black boxes show the duration of the respective phases according to elevation (see Table 1). X-axis represents months and DOY
The climatic conditions at the differing times of beginning (bENL) and cessation (cENL) of enlarging phases in each elevation belt vary strongly (Fig. 7). The influence of the elevation gradient is apparent for temperature and VPD for cENL. For bENL, there is a clear east–west separation for VPD. The differences between bENL and cENL are substantial, except for precipitation. There are higher precipitation amounts, lower temperatures and lower VPD during the beginning of the enlarging phases than during their endings. The beginning of tracheid enlargement (bENL) at the western coastal site (WL) could not be considered here due to its occurrence in the previous year. During the onset of ENL, the coastal site had the highest precipitation, but during the cessation of ENL, the two coastal sites are on opposite sides of the spectrum (Fig. 7A, D). The pattern for temperature is similar for the beginning and the end of the growing period: the higher elevation sites experience the lowest temperatures, and the lower sites have higher temperatures (Fig. 7B, E). Temperature and VPD show a clear east–west gradient during the cessation of the enlarging phase (Fig. 7E, F), but this is not the case during bENL (Fig. 7B, C). There are noticeable differences in (Fig. 7A–C) between species at mid-elevation sites EM and WM where they co-occur. Pinus nigra experienced slightly more precipitation, higher temperatures and higher VPD during bENL than P. pinaster.
Climate parameters (precipitation, temperature and VPD) for the beginning of the enlarging phase (DOY of bENL, upper panels) and its cessation (DOY of cENL, lower panels). Values of climate parameters are sums/means of 10 days before to 5 days after the modelled critical dates of beginning and cessation of each cell differentiation phase. Red solid lines show linear regressions, and vertical dashed lines indicate the mean value of all sites
Discussion
In this study, we quantified the growth dynamics of P. nigra and P. pinaster along an elevation gradient on Corsica. The change in elevation is clearly traceable at the beginning of cell enlargement and the growing season duration: trees at high elevations grow for a shorter time period than trees at lower elevations. But how is that influenced by climate? The attribution to a specific climate parameter and season often remains difficult due to sparse availability of long-term meteorological records, especially at high elevations. We addressed this problem by deploying meteorological stations in close proximity to our tree sites. Short-term but high-resolution investigations like our study can help to improve the interpretation of long-term data and provide a more complete picture of how trees react to climate variability.
Environmental factors at growth initiation and cessation
The beginning of wood formation in conifers is primarily controlled by temperature and photoperiod (Delpierre et al. 2019). Based on studies of cambial growth of Pinus cembra in the Austrian Alps, a temperature threshold of 5 °C is often assumed for initiating tree growth because of possible stem freezing below this threshold (Loris 1981; Gruber et al. 2009; Etzold et al. 2022). In our case study from Corsica, we observed an island-wide regional average 15-day mean temperature of 6.4 °C for the onset of cambial growth, but of only 3.1 °C for P. nigra at the highest elevation. In turn, it was 9.9 °C for P. pinaster at the eastern coast. Precipitation around the formation of the first cell varied even more, from 36.3 to 79.8 mm, with an island-wide average of 57 mm (Fig. 7).
Trees started growth according to the elevation gradient, but halt tracheid enlargement at most sites in September, except at the eastern coastal site (EL). Cessation of cambial division and cell expansion are guided by decreasing photoperiod and the onset of unfavourable conditions such as water deficit or temperature stress (De Micco et al. 2019). One indication for the reason being low temperatures is that the respective temperatures at cENL follow the elevation gradient (Fig. 7E). This corresponds to the high correlation of nENL with temperature (see Fig. 6). The P. pinaster trees at low-elevation site EL have access to deeper soil water (Häusser et al. 2021) and are an outlier from the elevation gradient (Fig. 7D–F) due to this strong influence of local site conditions.
Growing season length and species comparison
The growing season length differs considerably along the elevation gradient. Cambial growth in the Mediterranean region is assumed to occur for a more extended time period with lower growth rates than in temperate regions and growth rates are assumed to be the main driver for the final number of cells in a tree ring (Rathgeber 2019). These assumptions are only partly in concordance with our findings. The trees at the coastal sites indeed show the longest growing seasons at both study sites (EL and WL). However, their maximum growth rates are higher than that of the high-elevation site (EH) and the western mid-elevation site (WM). In addition, the final cell numbers do not exactly fit to the elevation gradient. The observation that more growth occurs on the eastern side of the island (EL and EM) was also found in a previous study using dendrometers (Häusser et al. 2021) and might be explained by more precipitation. In any case, the correlation between growth and elevation, or rather temperate and Mediterranean climate, seems not to be simplistic.
In the medium elevation belt of mixed forests, the growth period of P. pinaster is slightly shifted forward. A comparison of the cell phases shows the highest difference in CWT between mid-August and early September (Fig. 4). Due to the average difference of 30 days between bCWT and bMAT at these sites (Table 1), we conclude that P. nigra's CWT phase takes longer than that of P. pinaster. Due to a sampling interval of 10 to 14 days, we, hence, cannot exclude that P. nigra's CWT was, thus, occasionally counted twice.
Correlations between nENL and climate parameters
There is no clear elevation gradient in the correlations indicating that the dominant controlling factor shifts from temperature to moisture dependency. A tree-ring study on P. pinaster in central Spain showed that increasing growing season temperature had a negative effect on tree growth (Bogino and Bravo 2008). That is consistent with our study, although negative correlations with growth lasted shorter at the highest site and were longest at the lowest site. The correlation pattern is not as clear for VPD. The impact of VPD on tree growth and especially tree mortality is increasing due to anthropogenic climate change (McDowell et al. 2022). However, due to the high temporal variability of this parameter, the correlation patterns for nENL and VPD are more discontinuous than with temperature. Correlation coefficients of nENL and precipitation are only significant for very short periods over the growing season. Previous studies showed that water availability plays an important role in controlling cambial activity (Carvalho et al. 2015; De Micco et al. 2019) and recent studies on tree water uptake on Corsica have revealed that the Corsican pine trees use different water sources throughout the growing season (Szymczak et al. 2020; Juhlke et al. 2021). The relatively low correlation values with precipitation may, therefore, result from the fact that trees not just utilise precipitation during the growing season, but also winter snowmelt water at higher elevations and deep soil water reservoirs at coastal sites may play an important role.
From cell enlarging to cell-wall thickening
The correlations with climatic parameters of nENL are comparable to nCWT. The number of cells in the wall-thickening phase correlates the least with precipitation (positively and negatively), and there is no clear elevation gradient from changing temperature to moisture dependency. Generally, lignification is described as being sensitive to temperature (Donaldson 2001). The lag between ENL and CWT in conifers is around 1 month in temperate regions and longer in Mediterranean areas (Cuny et al. 2015). Depending on elevation, we found an offset of 45–53 days between the two phases. A distinctive feature is the nCWT at the coastal sites and their deviation from the pattern of the mid and high-elevation belts (Fig. 2). A reason for this divergence might be the relatively high daily minimum temperatures at the coasts, as cell-wall deposition rates during latewood formation are strongly constrained by temperature (Cuny and Rathgeber 2016).
According to our growth model, constant CWT occurs at the western coast (WL), which can be explained by the brief cambial dormancy period (Fig. 2). ENL already starts late in the previous year and ends late in the current year. The CWT pattern at low-elevation site EL is different because the growth seasonality at the coasts is shifted. Growth and CWT at the east coast start later, so the GAM is able to represent their onset at the beginning of the year. The chance of cambial reactivation at EL is much higher. This leads to a prolonged ENL and CWT phase. Our GAM does not depict the decline of wall-thickening cells due to this shifted growing season. The CWT phases at all sites (except WL) show a resurge in CWT.
Not all Mediterranean pine species show a distinct cambium dormancy if winter temperatures exceed the threshold of 9–10 °C (Prislan et al. 2016; Balzano et al. 2018). Pinus pinaster is particularly prone to cambial reactivation due to the production of wider tree rings compared to other pines (Zalloni et al. 2016). The starting threshold deduced in this study is 9.9 °C at the eastern coast in the first half of February which lead to cambial dormancy.
Conclusion
We were able to quantify and explain the high plasticity of radial growth patterns in two native Corsican pine species (Pinus nigra subsp. laricio and P. pinaster) along an elevation gradient spanning from Mediterranean to subalpine climate conditions. Accordingly, our first hypothesis was verified, since the growing season lengths decreased considerably along the studied elevation gradient. There is an approximately 1-month delay from the low to the mid-elevation belt and from the mid to the high-elevation belt for the appearance of first enlarging cells. The cessation of tissue formation occurs almost simultaneously at the mid-elevation and high-elevation sites. Contrary to our expectation, the modelled growth rates in the low-elevation belt are higher than at the highest site, leading us to reject our second hypothesis. The last hypothesis was partly rejected, since minimum temperatures for growth initiation under subalpine conditions can indeed be lower than the generally assumed 5 °C threshold. Moreover, coastal P. pinaster entered winter dormancy at temperatures around 9.9 °C, but exhibited a nearly constant state of cell-wall thickening. This difference clearly points to genetic adaptations of the two studied pine species to Mediterranean versus temperate climate conditions. This is confirmed by the fact that in the mid-elevation belt, where the two pine species co-occur, P. pinaster starts growth slightly earlier and mostly stops earlier than P. nigra, and that P. nigra exhibits higher radial growth rates than P. pinaster.
Additionally, we found that the growth seasonality of the two coastal P. pinaster sites is shifted: the enlarging phase in the west starts in December of the previous year, whilst it stops in late December of the current year. The tendency for cambial reactivation in pines after summer droughts was only apparent in the low-elevation belt.
Our study improves the understanding of differences in radial tree growth between mesic high-elevation and more xeric low-elevation sites in the Mediterranean. The tracheid cells investigated in this study not only provide insight into the ecological behaviour of two native Corsican pine species, but shed light on the climate–growth relationships of a widespread and ecologically and economically important genus in the Mediterranean and temperate regions. Our work lays the foundation for future studies to model predictions of radial growth and different climate conditions within this genus or with other genera across a broad environmental gradient.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Balzano A, Cufar K, Battipaglia G, Merela M, Prislan P, Aronne G, De Micco V (2018) Xylogenesis reveals the genesis and ecological signal of IADFs in Pinus pinea L. and Arbutus unedo L. Ann Bot 121:1231–1242
Bogino SM, Bravo F (2008) Growth response of Pinus pinaster Ait. to climatic variables in central Spanish forests. Ann for Sci 65:506
Carvalho A, Nabais C, Vieira J, Rossi S, Campelo F (2015) Plastic response of tracheids in Pinus pinaster in a water-limited environment: Adjusting lumen size instead of wall thickness. PLoS ONE 10:e0136305
Cocozza C, Palombo C, Tognetti R, La Porta N, Anichini M, Giovannelli A, Emiliani G (2016) Monitoring intra-annual dynamics of wood formation with microcores and dendrometers in Picea abies at two different altitudes. Tree Physiol 36:832–846
Cornes RC, van der Schrier G, van den Besselaar EJM, Jones PD (2018) An ensemble version of the E-OBS temperature and precipitation data sets. J Geophys Res Atmos 123:9391–9409
Cuny HE, Rathgeber CBK (2016) Xylogenesis: coniferous trees of temperate forests are listening to the climate tale during the growing season but only remember the last words! Plant Physiol 171:306–317
Cuny HE, Rathgeber CBK, Kiessé TS, Hartmann FP, Barbeito I, Fournier M (2013) Generalized additive models reveal the intrinsic complexity of wood formation dynamics. J Exp Bot 64:1983–1994
Cuny HE, Rathgeber CBK, Frank D, Fonti P, Mäkinen H, Prislan P, Rossi S, Del Castillo EM, Campelo F, Vavrčík H, Camarero JJ, Bryukhanova MV, Jyske T, Gričar J, Gryc V, de Luis M, Vieira J, Čufar K, Kirdyanov AV, Oberhuber W, Treml V, Huang J-G, Li X, Swidrak I, Deslauriers A, Liang E, Nöjd P, Gruber A, Nabais C, Morin H, Krause C, King G, Fournier M (2015) Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat Plants 1:15160
De Micco V, Carrer M, Rathgeber CB, Julio Camarero J, Voltas J, Cherubini P, Battipaglia G (2019) From xylogenesis to tree rings: wood traits to investigate tree response to environmental changes. IAWA 40:155–182
Delpierre N, Lireux S, Hartig F, Camarero JJ, Cheaib A, Čufar K, Cuny H, Deslauriers A, Fonti P, Gričar J, Huang J-G, Krause C, Liu G, de Luis M, Mäkinen H, Del Castillo EM, Morin H, Nöjd P, Oberhuber W, Prislan P, Rossi S, Saderi SM, Treml V, Vavrick H, Rathgeber CBK (2019) Chilling and forcing temperatures interact to predict the onset of wood formation in Northern Hemisphere conifers. Glob Chang Biol 25:1089–1105
Donaldson LA (2001) Lignification and lignin topochemistry—an ultrastructural view. Phytochemistry 57:859–873
Esper J, Hartl C, Konter O, Reinig F, Römer P, Huneau F, Lebre S, Szymczak S, Bräuning A, Büntgen U (2021) Past millennium hydroclimate variability from Corsican pine tree-ring chronologies. Boreas 51:621–636
Etzold S, Sterck F, Bose AK, Braun S, Buchmann N, Eugster W, Gessler A, Kahmen A, Peters RL, Vitasse Y, Walthert L, Ziemińska K, Zweifel R (2022) Number of growth days and not length of the growth period determines radial stem growth of temperate trees. Ecol Lett 25:427–439
Gao S, Liang E, Liu R, Babst F, Camarero JJ, Fu YH, Piao S, Rossi S, Shen M, Wang T, Peñuelas J (2022) An earlier start of the thermal growing season enhances tree growth in cold humid areas but not in dry areas. Nat Ecol Evol 6:397–404
Gričar J, Prislan P, Gryc V, Vavrčík H, de Luis M, Cufar K (2014) Plastic and locally adapted phenology in cambial seasonality and production of xylem and phloem cells in Picea abies from temperate environments. Tree Physiol 34:869–881
Gruber A, Baumgartner D, Zimmermann J, Oberhuber W (2009) Temporal dynamic of wood formation in Pinus cembra along the alpine treeline ecotone and the effect of climate variables. Trees 23:623–635
Gruber A, Strobl S, Veit B, Oberhuber W (2010) Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiol 30:490–501
Guijarro JA (2018) climatol: climate tools (series homogenization and derived products). http://www.climatol.eu/. Accessed 5 Dec 2022
Hastie T, Tibshirani R (1986) Generalized additive models. Stat Sci 1:297–310
Häusser M, Szymczak S, Garel E, Santoni S, Huneau F, Bräuning A (2019) Growth variability of two native pine species on Corsica as a function of elevation. Dendrochronologia 54:49–55
Häusser M, Szymczak S, Knerr I, Bendix J, Garel E, Huneau F, Trachte K, Santoni S, Bräuning A (2021) The dry and the wet case: tree growth response in climatologically contrasting years on the Island of Corsica. Forests 12:1175
Huang J-G, Zhang Y, Wang M, Yu X, Deslauriers A, Fonti P, Liang E, Mäkinen H, Oberhuber W, Rathgeber CBK, Tognetti R, Treml V, Yang B, Zhai L, Zhang J-L, Antonucci S, Bergeron Y, Camarero JJ, Campelo F, Čufar K, Cuny HE, de Luis M, Fajstavr M, Giovannelli A, Gričar J, Gruber A, Gryc V, Güney A, Jyske T, Kašpar J, King G, Krause C, Lemay A, Liu F, Lombardi F, Del Castillo EM, Morin H, Nabais C, Nöjd P, Peters RL, Prislan P, Saracino A, Shishov VV, Swidrak I, Vavrčík H, Vieira J, Zeng Q, Liu Y, Rossi S (2023) A critical thermal transition driving spring phenology of Northern Hemisphere conifers. Glob Change Biol 29:1606–1617
IPCC (2022) Climate Change 2022: impacts, adaptation and vulnerability: contribution of working group ii to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, UK and New York, NY, USA
Jevšenak J, Gričar J, Rossi S, Prislan P (2022) Modelling seasonal dynamics of secondary growth in R. Ecography 9:1–8
Juhlke TR, van Geldern R, Barth JAC, Bendix J, Bräuning A, Garel E, Häusser M, Huneau F, Knerr I, Santoni S, Szymczak S, Trachte K (2021) Temporal offset between precipitation and water uptake of Mediterranean pine trees varies with elevation and season. Sci Total Environ 755:142539
Knerr I, Trachte K, Garel E, Huneau F, Santoni S, Bendix J (2020) Partitioning of large-scale and local-scale precipitation events by means of spatio-temporal precipitation regimes on Corsica. Atmosphere 11:417
Krepkowski J, Bräuning A, Gebrekirstos A, Strobl S (2011) Cambial growth dynamics and climatic control of different tree life forms in tropical mountain forest in Ethiopia. Trees 25:59–70
Loris K (1981) Dickenwachstum von Zirbe, Fichte und Lärche an der alpinen Waldgrenze/Patscherkofel. Mittl Forstl Bundesversuchsanstalt 142:417–441
McDowell NG, Sapes G, Pivovaroff A, Adams HD, Allen CD, Anderegg WRL, Arend M, Breshears DD, Brodribb T, Choat B, Cochard H, de Cáceres M, de Kauwe MG, Grossiord C, Hammond WM, Hartmann H, Hoch G, Kahmen A, Klein T, Mackay DS, Mantova M, Martínez-Vilalta J, Medlyn BE, Mencuccini M, Nardini A, Oliveira RS, Sala A, Tissue DT, Torres-Ruiz JM, Trowbridge AM, Trugman AT, Wiley E, Xu C (2022) Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit. Nat Rev Earth Environ 3:294–308
MétéoFrance (2020a) A data set from the Climate stations Ajaccio, Bocognano, Evisa, Sampolo and Solenzara. https://www.meteofrance.fr. Accessed 20 May 2020
MétéoFrance (2020b) Bulletins climatiques de france métropolitaine et outre-mer. https://donneespubliques.meteofrance.fr/?fond=produit&id_produit=129&id_rubrique=29. Accessed 6 July 2020
Prislan P, Gričar J, de Luis M, Novak K, Del Martinez CE, Schmitt U, Koch G, Štrus J, Mrak P, Žnidarič MT, Čufar K (2016) Annual cambial rhythm in Pinus halepensis and Pinus sylvestris as indicator for climate adaptation. Front Plant Sci 7:1923
Pumijumnong N, Songtrirat P, Buajan S, Preechamart S, Chareonwong U, Muangsong C (2021) Climate control of cambial dynamics and tree-ring width in two tropical pines in Thailand. Agric Meteorol 303:108394
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/. Accessed 20 Nov 2022
Rathgeber C (2019) Ecophysiology of tree-ring formation: concepts, methods and applications. Université de Lorraine (Nancy)
Rathgeber CBK, Cuny HE, Fonti P (2016) Biological basis of tree-ring formation: a crash course. Front Plant Sci 7:734
Rathgeber CBK, Santenoise P, Cuny HE (2018) CAVIAR: an R package for checking, displaying and processing wood-formation-monitoring data. Tree Physiol 38:1246–1260
Ren P, Rossi S, Camarero JJ, Ellison AM, Liang E, Peñuelas J (2018) Critical temperature and precipitation thresholds for the onset of xylogenesis of Juniperus przewalskii in a semi-arid area of the north-eastern Tibetan Plateau. Ann Bot 121:617–624
Römer P, Hartl C, Schneider L, Bräuning A, Szymczak S, Huneau F, Lebre S, Reinig F, Büntgen U, Esper J (2021) Reduced temperature sensitivity of maximum latewood density formation in high-elevation corsican pines under recent warming. Atmosphere 12:804
Rossi S, Deslauriers A, Morin H (2003) Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia 21:33–39
Rossi S, Deslauriers A, Anfodillo T (2006a) Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the alpine timberline. IAWA J 27:383–394
Rossi S, Menardi R, Anfodillo T (2006b) Trephor: a new tool for sampling microcores from tree stems. IAWA J 27:89–97
RStudio Team (2021) RStudio: Integrated development for R. https://www.rstudio.com/. Accessed 20 Nov 2022
Simpson GL (2022) gratia: graceful ggplot-based graphics and other functions for GAMs fitted using mgcv. https://gavinsimpson.github.io/gratia/. Accessed 7 Dec 2022
Szymczak S, Barth J, Bendix J, Huneau F, Garel E, Häusser M, Juhlke T, Knerr I, Santoni S, Mayr C, Trachte K, van Geldern R, Bräuning A (2020) First indications of seasonal and spatial variations of water sources in pine trees along an elevation gradient in a Mediterranean ecosystem derived from δ18O. Chem Geol 549:119695
Versace S, Bräuning A, Cherubini P, Di Febbraro M, Häusser M, Lombardi F, Marchetti M, Marziliano PA, Salbitano F, Szymczak S, Tognetti R (2022) New evidence for population-specific responses to drought events from tree ring chronologies of Pinus nigra ssp. laricio across the entire distribution range. Agric for Meteorol 323:109076
Vieira J, Carvalho A, Campelo F (2020) Tree growth under climate change: evidence from xylogenesis timings and kinetics. Front Plant Sci 11:90
Wilson BF, Wodzicki TJ, Zahner R (1966) Differentiation of cambial derivatives: proposed terminology. For Sci 12:438–440
WMO (2018) Guide to meteorological instruments and methods of observation: Part I. Measurement of meteorological variables. Chapter 4—measurement of humidity, vol 8. World Meteorological Organization, Geneva
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodol 73:3–36
Zalloni E, de Luis M, Campelo F, Novak K, De Micco V, Di Filippo A, Vieira J, Nabais C, Rozas V, Battipaglia G (2016) Climatic signals from intra-annual density fluctuation frequency in mediterranean pines at a regional scale. Front Plant Sci 7:579
Acknowledgements
We thank Kim-Alana Holz and Salomon Zevallos for their assistance with the extensive laboratory work needed to carry out this study. Furthermore, we acknowledge MétéoFrance for supplying the climate data.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by the German Research Foundation (Grant numbers BE 1780/45-1, BR 1895/27-1, GE 2338/1-1, SZ 356/1-1 and TR 1201/2-1) as part of the interdisciplinary research programme CorsicArchive (Altitudinal Gradients and Forest Response: Climate, Hydrology and Isotope Variability of a Mediterranean Ecosystem) DFG-PAK 927/1.
Author information
Authors and Affiliations
Contributions
Study conception and design: MH and AB; methodology implementation and data collection: MH, JACB, JB, EG, RG, FH, TRJ, IK, SSa, SSz, KT and AB; data analysis and interpretation: MH, SA and AB; manuscript writing/revision: MH, SA, JACB, JB, EG, RG, FH, TRJ, IK, SSa, SSz, KT and AB.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by S. Rossi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
468_2023_2402_MOESM2_ESM.tif
Supplementary Figure S2: Standardised cell counts (P. nigra: upward pointing trinagle, P. pinaster: downward pointing triangle) and modelled cell numbers (lines) for the enlarging (ENL), cell wall thickening (CWT), and mature (MAT) phases. (TIF 6040 KB)
468_2023_2402_MOESM3_ESM.xlsx
Supplementary Table S1: GAM statistics for the enlarging (ENL), cell wall thickening (CWT), and mature (MAT) phases of each tree. (XLSX 19 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Häusser, M., Aryal, S., Barth, J.A.C. et al. Xylem formation patterns from Mediterranean to subalpine climate conditions reveal high growth plasticity of pine species on Corsica. Trees 37, 1027–1039 (2023). https://doi.org/10.1007/s00468-023-02402-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02402-8