Abstract
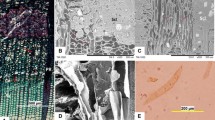
The effect of a short-term low temperature on cellular characteristics of a frost ring in radiata pine (Pinus radiata) secondary xylem was investigated using various microscopic techniques. Cell walls in the frost ring, that formed in the earlywood due to an abrupt drop in the temperature one night in the Spring, were poorly developed, lacking in the proper thickness and the proportion of wall constituents. In majority of the cases, the cell walls were highly convoluted and the secondary walls developed poorly and incompletely. Judging by irregular deposition of lignin, it appears that the control mechanism ensuring an orderly deposition of monolignols failed to function properly. The highly porous texture of some secondary walls indicated that cellulosic and hemicellulosic framework was affected, which would explain irregular lignification of cell walls. Thus the frost ring constitutes a serious defect in the timber, being a zone of weakness along which the wood is likely to split during processing, such as drying.














Similar content being viewed by others
References
Aroca R, Amodeo G, Fernendez-Illescas S, Herman EM, Chaumont F, Chrispeels MJ (2005) The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol 137:341–353
Barnett JR (1976) Rings of collapsed cells in Pinus radiata stemwood from lysimeter-grown trees subjected to drought. New Zealand J For Sci 6:461–465
Bray EA (2004) Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. J Exp Bot 55:2331–2341
Brummell DA, Cin VD, Lurie S, Crisosto CH, Labavitch JM (2004) Cell wall metabolism during the development of chilling injury in cold-stored peach fruit: association of mealiness with arrested disassembly of cell wall pectins. J Exp Bot 55:2041–2052
Cano-Delgado A, Penfield S, Smith C, Catley M, Bevan M (2003) Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J 34:351–362
Chang S, Puryear JD, Dias MADL, Funkhouser EA, Newton RJ, Cairney J (1995) Gene expression under water deficit in loblolly pine (Pinus taeda L.): isolation and characterization of cDNA clones. Physiol Plant 95:1–10
Cipollini DF Jr (1998) The induction of soluble peroxidase activity in bean leaves by wind-induced mechanical perturbation. Am J Bot 85:1586–1591
Costa P, Bahrman N, Frigerio JM, Kremer A, Plomion C (1998) Water-deficit-responsive proteins in maritime pine. Plant Mol Biol 38:587–596
Creelman RA, Mullet JE (1991) Water deficit modulates gene expression in growing zones of soybean seedlings. Analysis of differentially expressed cDNA, a new beta-tubulin gene, and expression of genes encoding cell wall proteins. Plant Mol Biol 17:591–608
Donaldson LA (2002) Abnormal lignin distribution in wood from severely drought stressed Pinus radiata trees. IAWA J 23:161–178
Donaldson LA (1992) Lignin distribution during latewood formation in Pinus radiata D. Don. IAWA Bull New Ser 13:381–387
Downes GM, Ward JV, Turvey ND (1991) Lignin distribution across tracheid cell walls of poorly lignified wood from deformed copper deficient Pinus radiata D. Don. Wood Sci Technol 25:7–14
Glerum C, Farrar JL (1966) Frost ring formation in the stem of some coniferous species. Can J Bot 44:879–886
Hafren J, Fujino T, Itoh T (1999) Changes in cell wall architecture of differentiating tracheids of Pinus thunbergii during lignification. Plant Cell Physiol 40:532–541
Hawes D, Satiat-Jeunemaitre B (2001) Plant cell biology, 2nd edn. Oxford University Press, Oxford
Jbir N, Chaibi W, Ammar S, Jemmali A, Ayadi A (2001) Root growth and lignification of two wheat species differing in their sensitivity to NaCl, in response to salt stress. CR Acad Sci Paris Sciences de la vie/Life Sci 324:863–868
Lee SH, Singh AP, Chung GC, Kim YS, Kong IB (2002) Chilling root temperature causes rapid ultrastructural changes in cortical cells of cucumber (Cucumis sativus L.) root tips. J Exp Bot 53:2225–2237
Lee SH, Singh AP, Chung GC (2004) Rapid accumulation of hydrogen peroxide in cucumber roots due to exposure to low temperature appears to mediate decreases in water transport. J Exp Bot 55:1733–1741
Lee SH, Chung GC, Steudle E (2005) Gating of aquaporins by low temperature in roots of chilling-sensitive cucumber and chilling-tolerant figleaf gourd. J Exp Bot 56:985–995
Nishida I, Murata N (1996) Chilling sensitivity in plants and cyanobacteria: the crucial distribution of membrane lipids. Annu Rev Plant Physiol Plant Mol Biol 47:541–568
Noguiera FTS, Rosa VED Jr, Menossi M, Ulian EC, Arruda P (2003) RNA expression profiles and data mining of sugarcane response to low temperature. Plant Physiol 132:1811–1824
Ruel K, Comtat J, Barnoud F (1977) Localization histologiquie et ultrastrurale des xylanes dans es parios primaries des tissues d’Arundo donax. C R Acad Sci Paris, Serie D 1421–1424
Schmitt U, Singh AP, Frankenstein C, Moller R (2006) Cell wall modifications in woody stems as a consequence of various causes. New Zealand J For Sci 36:72–86
Shigo AG (1979) Tree decay: an expanded concept. US Dept. Agr. Agr. Inf. Bull. 419. 73 pp
Terashima N (2000) Formation and ultrastructure of lignified plant cell walls. In: Kim YS (ed) New horizons in wood anatomy. Chonnam National University Press, Kwangju, pp 169–180
Thiery JP (1967) Mise en evidence des polysaccharides sur coupes fines en microscopie electronique. J Microsc 6:987–1018
Timell TE (1986) Compression wood in gymnosperms, vol 1. Springer, Heidelberg
Toquin V, Grausem B, Geoffroy P, Legrand M (2003) Structure of the tobacco caffeic acid O-methyltrasferase (COMT) II gene: identification of promoter sequences involved in gene inducibility by various stimuli. Plant Mol Biol 52:495–509
Vincent D, Lapierre C, Pollet B, Cornic G, Negroni L, Zivy M (2005) Water deficits affect caffeate O-methyltransferase, lignification, and related enzymes in maize leaves. A proteomic investigation. Plant Physiol 137:949–960
Wi SG, Singh AP, Lee KH, Kim YS (2005) The pattern of distribution of pectin, peroxidase and lignin in the middle lamella of secondary xylem fibres in alfalfa (Medicago sativa). Ann Bot 95:863–868
Zhong H, Lauchli A (1993) Changes in cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. J Exp Bot 44:773–778
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF grant no. R01-2003-000-10073-0). L.K.H. is grateful for BK21 program provided by the Ministry of Education. Authors thank Dr. Peter Beets of Ensis in Rotorua, New Zealand, for his help in obtaining the samples. They are also very grateful for comments of anonymous reviewer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Buckeridge.
Rights and permissions
About this article
Cite this article
Lee, K.H., Singh, A.P. & Kim, Y.S. Cellular characteristics of a traumatic frost ring in the secondary xylem of Pinus radiata . Trees 21, 403–410 (2007). https://doi.org/10.1007/s00468-007-0131-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-007-0131-5