Abstract
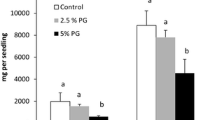
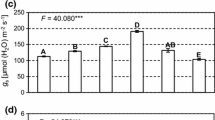
Hybridisation between certain willow species is a common feature leading to novel genotypes varying in growth rate and stress tolerance. The objective of this 4-week study was to investigate the effects of decreased watering, enhanced ultraviolet-B irradiation (UV-BBE, 280–315 nm, 7.2 kJ m−2 day−1) and combined decreased watering and enhanced UV-B irradiation on di- and polyamines in the leaves of Salix myrsinifolia and its hybrid with S. myrsinites. Control plantlets were well-watered and exposed to ambient UV-B irradiation (UV-BBE, 3.6 kJ m−2 day−1). HPLC analyses showed that the constitutive concentrations of soluble di- and polyamines varied markedly between S. myrsinifolia and its hybrids. The degree of responses to treatments also varied: in S. myrsinifolia, concentrations of free putrescine were clearly increased by reduced watering, while in the hybrid willow, change in putrescine was less pronounced and not significant. Results also showed that the increase in putrescine in S. myrsinifolia by reduced watering was mitigated by concurrent enhancement of UV-B irradiation. There were no direct UV-B effects on the soluble polyamines.


Similar content being viewed by others
References
Aziz A, Martin-Tanguy J, Larher F (1997) Plasticity of polyamine metabolism associated with high osmotic stress in rape leaf discs and with ethylene treatment. Plant Growth Regul 21:153–163
Björn LO (1990) Photobiology, Second Book. University of Lund, Lund, pp 55–59
Björn LO, Callaghan TV, Johnsen I, Lee JA, Manetas Y, Paul ND, Sonesson M, Wellburn AR, Coop D, Heide-Jørgensen HS, Gehrke C, Gwynn-Jones D, Johanson U, Kyparissis A, Levizou E, Nikolopoulos D, Petropoulou Y, Stephanou M (1997) The effects of UV-B radiation on European heathland species. Plant Ecol 128:253–264
Bouchereau A, Aziz A, Larher F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125
Caldwell MM, Gold WG, Harris G, Ashurst CW (1983) A modulated lamp system for solar UV-B (280–320 nm) supplementation studies in the field. Photochem Photobiol 37:479–485
DiTomaso JM, Shaff JE, Kochian LV (1989) Putrescine-induced wounding and its effects on membrane integrity and ion transport processes in roots of intact corn seedlings. Plant Physiol 90:988–995
Finnish Meteorological Institute (1993) Measurements of solar radiation 1981–90. Meteorological Yearbook of Finland, vol 1, Part 4, pp 81–91.
Flores HE, Galston AW (1982a) Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol 69:701–706
Flores HE, Galston AW (1982b) Polyamines and plant stress: activation of putrescine biosynthesis by osmotic shock. Science 217:1259–1260
Hämet-Ahti L, Suominen J, Ulvinen T, Uotila P (eds) (1998) Retkeilykasvio (Field Flora of Finland), 4th edn. Finnish Museum of Natural History, Botanical Museum, Helsinki, Finland
Heby O (1981) Role of polyamines in the control of cell proliferation and differentiation. Differentiation 19:1–12
Heiskanen J (1994) Hydrological properties of peat-based growth media. PhD Thesis. Metsäntutkimuslaitoksen tiedonantoja 524, Finnish Forest Research Institute, Suonenjoki Research Station, Finland
Hideg É, Nagy T, Oberschall A, Dudits D, Vass I (2003) Detoxification function of aldose/aldehyde reductase during drought and ultraviolet-B (280–320 nm) stresses. Plant Cell Environ 26:513–522
Hofmann RW, Campbell BD, Bloor SJ, Swinny EE, Markham KR, Ryan KG, Fountain DW (2003) Responses to UV-B radiation in Trifolium repens L.—physiological links to plant productivity and water availability. Plant Cell Environ 26:603–612
Kramer GF, Norman HA, Krizek DT, Mirecki RM (1991) Influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochemistry 30:2101–2108
Kramer GF, Krizek DT, Mirecki RM (1992) Influence of photosynthetically active radiation and spectral quality on UV-B-induced polyamine accumulation in soybean. Photochemistry 31:1119–1125
Krizek DT, Kramer GF, Upadhyaya A, Mirecki RM (1993) UV-B response of cucumber seedlings grown under metal halide and high pressure sodium/deluxe lamps. Physiol Plant 88:350–358
Liu K, Fu HH, Bei QX, Luan S (2000) Inward potassium channel in guard cells as targets for polyamine regulation of stomatal movements. Plant Physiol 124:1315–1325
Liu HP, Dong BH, Zhang YY, Liu ZP, Liu YL (2004) Relationships between osmotic stress and the levels of free, conjugated and bound polyamines in leaves of wheat seedlings. Plant Sci 166:1261–1267
Logothetis K, Dakanali S, Ioannidis N, Kotzabasis K (2004) The impact of high CO2 concentrations on the structure and function of the photosynthetic apparatus and the role of polyamines. J Plant Phys 161:715–724
Martin-Tanguy J (1997) Conjugated polyamines and reproductive development: biochemical, molecular and physiological approaches. Physiol Plant 100:675–688
Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul 34:135–148
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Paschalidis KA, Roubelakis-Angelakis KA (2005) Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs/tissues of the tobacco plant. Correlations with age, cell division/expansion, and differentiation. Plant Physiol 138:142–152
Predieri S, Krizek DT, Wang CY, Mirecki RM, Zimmerman RH (1993) Influence of UV-B radiation on developmental changes, ethylene, CO2 flux and polyamines in cv. Doyenne d’Hiver pear shoots grown in vitro. Physiol Plant 87:109–117
Reich PB, Walters MB, Ellsworth DS (1992) Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol Monogr 62:365–392
Skvortsov AK (1999) Willows of Russia and adjacent countries. University of Joensuu, Faculty of Mathematics and Natural Sciences, Report Series no:39, Biology, Joensuu, Finland
Smith J, Burritt D, Bannister P (2001) Ultraviolet-B radiation leads to a reduction in free polyamines in Phaseolus vulgaris L. Plant Growth Regul 35:289–294
Turtola S, Rousi M, Pusenius J, Yamaji K, Heiska S, Tirkkonen V, Meier B, Julkunen-Tiitto R (2005a) Genotypic variation in drought response of willows grown under ambient and enhanced UV-B radiation. Doi:10.1016/j.enexpbot.2005.01.007
Turtola S, Rousi M, Pusenius J, Yamaji K, Heiska S, Tirkkonen V, Meier B, Julkunen-Tiitto R (2005b) Clone-specific responses in leaf phenolics of willows exposed to enhanced UVB radiation and drought stress. Doi: 10.1111/j.1365-2486.2005.01013x
Walden R, Cordiero C, Tiburcio F (1997) Polyamine: small molecules triggering pathways in plant growth and development. Plant Physiol 113:1009–1013
Acknowledgements
This work was supported by the Academy of Finland (project no. 76681). We also thank Sinikka Sorsa and Maija Makkonen for their help with the micropropagations, and Hanni Sikanen, Mari Tuomainen and Keiko Yamaji for their assistance during the experiment in Punkaharju.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Hampp
Rights and permissions
About this article
Cite this article
Tegelberg, R., Turtola, S., Rousi, M. et al. Soluble polyamines in Salix myrsinifolia and S. myrsinites × S. myrsinifolia plantlets exposed to increased UV-B irradiation and decreased watering. Trees 20, 299–303 (2006). https://doi.org/10.1007/s00468-005-0036-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-005-0036-0