Abstract
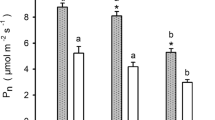
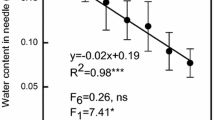
Rates of photosynthesis vary with foliage age and typically decline from full-leaf expansion until senescence occurs. This age-related decline in photosynthesis is especially important in species that retain foliage for several years, yet it is not known whether the internal conductance to CO2 movement (g i) plays any role. More generally, g i has been measured in only a few conifers and has never been measured in leaves or needles older than 1 year. The effect of ageing on g i was investigated in Pinus pinaster, a species that retains needle for 4 or more years. Measurements were made in autumn when trees were not water limited and after leaf expansion was complete. Rates of net photosynthesis decreased with needle age, from 8 μmol m−2 s−1 in fully expanded current-year needles to 4.4 μmol m−2 s−1 in 3-year-old needles. The relative limitation due to internal conductance (0.24–0.35 out of 1) was in all cases larger than that due to stomatal conductance (0.13–0.19 out of 1). Internal conductance and stomatal conductance approximately scaled with rates of photosynthesis. Hence, there was no difference among year-classes in the relative limitations posed by internal and stomatal conductance or evidence that they cause the age-related decline in photosynthesis. There was little evidence that the age-related decline in photosynthesis was due to decreases in contents of N or Rubisco. The decrease in rates of photosynthesis from current-year to older needles was instead related to a twofold decrease in rates of photosynthesis per unit nitrogen and V cmax/Rubisco (i.e., in vivo specific activity).



Similar content being viewed by others
References
Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130:1–7
Canvin DT, Berry JA, Badger MR, Fock H, Osmond CB (1980) Oxygen exchange in leaves in the light. Plant Physiol 66:302–307
Centritto M, Loreto F, Chartzoulakis K (2003) The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant Cell Environ 26:585–594
De Lucia EH, Whitehead D, Clearwater MJ (2003) The relative limitation of photosynthesis by mesophyll conductance in co-occurring species in a temperate rainforest dominated by the conifer Dacrydium cupressinum. Funct Plant Biol 30:1197–1204
Epron D, Godard D, Cornic G, Genty B (1995) Limitation of net CO2 assimilation rate by internal resistance to CO2 transfer in the leaves of two tree species (Fagus sylvatica L. and Castanea sativa Mill.). Plant Cell Environ 18:43–51
Ethier GJ, Livingston NJ (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ 27:137–153
Evans JR, von Caemmerer S, Setchell BA, Hudson GS (1994) The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Aust J Plant Physiol 21:475–495
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Field C (1983) Allocating leaf nitrogen for the maximisation of carbon gain: leaf age as a control on the allocation program. Oecologia 56:341–347
Freeland RO (1952) Effect of age of leaves upon the rate of photosynthesis in some conifers. Plant Physiol 27:685–690
Gillon JS, Yakir D (2000) Internal conductance to CO2 diffusion and (COO)-O-18 discrimination in C-3 leaves. Plant Physiol 123:201–2
Hanba YT, Miyazawa S-I, Kogami H, Terashima I (2001) Effects of leaf age on internal CO2 transfer conductance and photosynthesis in tree species having different types of shoot phenology. Aust J Plant Physiol 28:1075–1084
Harley PC, Loreto F, Di Marco G, Sharkey TD (1992) Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol 98:1429–1436
Hidema J, Makino A, Mae T, Ojima K (1991) Photosynthetic characteristics of rice leaves aged under different irradiances from full expansion through to senescence. Plant Physiol 97:1287–1293
Jurik TW (1986) Seasonal patterns of leaf photosynthetic capacity in successional northern hardwood tree species. Am J Bot 73:131–138
Laisk AK (1977) Kinetics of photosynthesis and photorespiration in C3-plants. Nauka, Moscow
Loreto F, Di Marco G, Tricoli D, Sharkey TD (1994) Measurements of mesophyll conductance, photosynthetic electron transport and alternative electron sinks of field grown wheat leaves. Photosynth Res 41:397–403
Miyazawa S-I, Terashima I (2001) Slow development of leaf photosynthesis in an evergreen broad-leaved tree, Castanopsis sieboldii: relationships between leaf anatomical characteristics and photosynthetic rate. Plant Cell Environ 24:279–291
Niinemets Ü (1997) Acclimation to low irradiance in Picea abies: influences of past and present light climate on foliage structure and function. Tree Physiol 17:723–732
Okada K, Katoh K (1992) Effects of light on degradation of chlorophyll and chloroplast proteins during senescence of rice leaves. In: Murata N (ed) Research in photosynthesis. Kluwer, Dorddrecht, pp 341–344
Peisker M, Apel H (2001) Inhibition by light of CO2 evolution from dark respiration: Comparison of two gas exchange methods. Photosynth Res 70:291–298
Pepin S, Livingston NJ, Whitehead D (2002) Responses of transpiration and photosynthesis to reversible changes in photosynthetic foliage area in western red cedar (Thuja plicata) seedlings. Tree Physiol 22:363–371
Scartazza A, Lauteri M, Guido MC, Brugnoli E (1998) Carbon isotope discrimination in leaf and stem sugars, water use efficiency and mesophyll conductance during different developing stages in rice subjected to drought. Aust J Plant Physiol 25:489–498
Šest k Z (1985) Photosynthesis during leaf development. Dr W. Junk Publishers, The Hague
Stitt M, Schulze E-D (1994) Does Rubisco control the rate of photosynthesis and plant growth? An exercise in molecular ecophysiology. Plant Cell Environ 17:465–487
Syvertsen JP, Lloyd J, McConchie C, Kriedemann PE, Farquhar GD (1995) On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves. Plant Cell Environ 18:149–157
Terashima I, Ono K (2002) Effects of HgCl2 on CO2 dependence of leaf photosynthesis: evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant Cell Physiol 43:70–78
von Caemmerer S, Evans JR (1991) Determination of the average partial pressure of CO2 in chloroplasts from leaves of several C3 plants. Aust J Plant Physiol 18:287–305
von Caemmerer S, Evans JR, Hudson GS, Andrews TJ (1994) The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta 195:88–97
Vitousek PM, Field CB, Matson PA (1990) Variation in foliar δ13C in Hawaiian Metrosideros polymorpha: a case of internal resistance? Oecologia 84:362–370
Warren CR (2004) The photosynthetic limitation posed by internal conductance to CO2 movement is increased by nutrient supply. J Exp Bot 406:2313–2321
Warren CR, Adams MA (2001) Distribution of N, Rubisco and photosynthesis in Pinus pinaster and acclimation to light. Plant Cell Environ 24:597–609
Warren CR, Adams MA, Chen ZL (2000) Is photosynthesis related to concentrations of nitrogen and Rubisco in leaves of Australian native plants? Aust J Plant Physiol 27:407–416
Warren CR, Ethier GJ, Livingston NJ, Grant NJ, Turpin DH, Harrison DL, Black TA (2003a) Transfer conductance in second growth Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) canopies. Plant Cell Environ 26:1215–1227
Warren CR, Dreyer E, Adams MA (2003b) Photosynthesis-Rubisco relationships in foliage of Pinus sylvestris in response to nitrogen supply and the proposed role of Rubisco and amino acids as nitrogen stores. Trees 17:359–366
Warren CR, Adams MA (2004) Evergreen trees do not maximize photosynthesis. Trends Plant Sci 9:270–274
Warren CR, Livingston NJ, Turpin DH (2004) Water stress decreases transfer conductance of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) seedlings. Tree Physiol 24:971–979
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Acknowledgements
The Australian Research Council is acknowledged for financial support. Frank Jones is thanked for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warren, C.R. Why does photosynthesis decrease with needle age in Pinus pinaster?. Trees 20, 157–164 (2006). https://doi.org/10.1007/s00468-005-0021-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-005-0021-7
Keywords
- Age
- Photosynthesis
- Nitrogen
- Internal resistance
- Mesophyll conductance
- Transfer conductance
- V cmax
- J max
- Rubisco
Abbreviations
- A:Rate of net photosynthesis
- Ail:Light-saturated rate of photosynthesis at Cc=Ci
- An:Rate of light-saturated net photosynthesis at a Ca=360 μmol mol−1
- Asl:Light-saturated rate of photosynthesis at Ci=360 μmol mol−1
- Ca:Ambient CO2 concentration
- Cc:Chloroplastic CO2 concentration
- Ci:Intercellular CO2 concentration
- Ci*:Intercellular photocompensation point
- Chl:Chlorophyll
- Γ*:Chloroplastic photocompensation point
- gi:Internal conductance to CO2
- gs:Stomatal conductance to water
- J:Rate of electron transport
- Jmax:Maximum rate of electron transport
- Li:Relative limitation of photosynthesis due to internal conductance
- Ls:Relative limitation of photosynthesis due to stomatal conductance
- N:Nitrogen
- PNUE:instantaneous photosynthetic nitrogen-use efficiency
- PPFD:photosynthetic photon flux density
- Rd:Rate of mitochondrial respiration in the light
- SLA:Specific leaf area (projected area/dry mass)
- Vcmax:Maximum rate of carboxylation