Abstract
Background
The use of genetic testing in pediatric patients with chronic kidney diseases (CKD) has increased exponentially in the past few years, particularly with the emergence of novel sequencing techniques. However, the genetic yield remains unexpectedly low in nephrology, with an impact on diagnosis, prognosis and treatment. Moreover, the increasing diversity of genetic testing possibilities can be seen as an obstacle by clinicians, in the absence of a strong background in genetics. Here, we propose a step-by-step, multidisciplinary strategy for the diagnostic evaluation of pediatric patients with CKD, and appropriate genetic test selection to maximize the yield of genetic testing.
Methods
A total of 126 pediatric patients were enrolled in a retrospective file analysis. Genetic testing techniques used included phenotype-associated next-generation panel sequencing (N = 41), Sanger and SNaPshot sequencing (N = 3) and/or whole exome sequencing (N = 2).
Results
Overall genetic yield reached 63% and genetic testing significantly impacted patient management in 70%. The distribution of kidney diseases among patients was balanced and matched previously described pediatric cohorts in terms of glomerulopathies, tubulopathies and ciliopathies. Genetic analyses led to significant treatment modifications, kidney biopsy sparing and personalized nephroprotection, as well as tailored genetic counseling. Of note, the evaluation of Human Phenotype Ontology term accuracy in the cohort showed that causal mutations were precisely identified in 85% of the patients at most.
Conclusion
Here we suggest a step-by-step, multidisciplinary strategy to maximize the yield of genetic testing in pediatric patients with CKD. This approach optimizes patient care while avoiding unnecessary treatments or procedures.
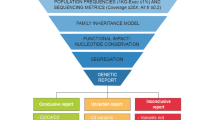
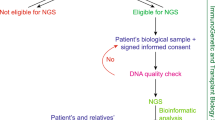
Graphical Abstract

A higher resolution version of the Graphical abstract is available as Supplementary information


Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to European privacy laws (GDPR), but are available from the corresponding author on reasonable request.
References
Chen J, Lin F, Zhai Y et al (2021) Diagnostic and clinical utility of genetic testing in children with kidney failure. Pediatr Nephrol 36:3653–3662. https://doi.org/10.1007/s00467-021-05141-5
Arora V, Anand K, Chander Verma I (2020) Genetic testing in pediatric kidney disease. Indian J Pediatr 87:706–715. https://doi.org/10.1007/s12098-020-03198-y
KDIGO Conference Participants (2022) Genetics in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 101:1126–1141. https://doi.org/10.1016/j.kint.2022.03.019
Vivante A, Hildebrandt F (2016) Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol 12:133–146. https://doi.org/10.1038/nrneph.2015.205
Gambaro G, Zaza G, Citterio F, Naticchia A, Ferraro PM (2019) Living kidney donation from people at risk of nephrolithiasis, with a focus on the genetic forms. Urolithiasis 47:115–123. https://doi.org/10.1007/s00240-018-1092-4
Pinto e Vairo F, Kemppainen JL, Lieske JC, Harris PC, Hogan MC (2021) Establishing a nephrology genetic clinic. Kidney Int 100:254–259. https://doi.org/10.1016/j.kint.2021.05.008
Alkanderi S, Yates LM, Johnson SA, Sayer JA (2017) Lessons learned from a multidisciplinary renal genetics clinic. QJM 110:453–457. https://doi.org/10.1093/qjmed/hcx030
Jayasinghe K, Quinlan C, Stark Z, Patel C, Mallawaarachchi A, Wardrop L, Kerr PG, Trnka P, Mallett AJ (2019) Renal genetics in Australia: kidney medicine in the genomic age. Nephrology 24:279–286. https://doi.org/10.1111/nep.13494
Jayasinghe K, Stark Z, Kerr PG, Gaff C, Martyn M, Whitlam J, Creighton B, Donaldson E, Hunter M, Jarmolowicz A, Johnstone L, Krzesinski E, Lunke S, Lynch E, Nicholls K, Patel C, Prawer Y, Ryan J, See EJ, Talbot A, Trainer A, Tytherleigh R, Valente G, Wallis M, Wardrop L, West KH, White SM, Wilkins E, Mallett AJ, Quinlan C (2021) Clinical impact of genomic testing in patients with suspected monogenic kidney disease. Genet Med 23:183–191. https://doi.org/10.1038/s41436-020-00963-4
Schrezenmeier E, Kremerskothen E, Halleck F, Staeck O, Liefeldt L, Choi M, Schüler M, Weber U, Bachmann N, Grohmann M, Wagner T, Budde K, Bergmann C (2021) The underestimated burden of monogenic kidney disease in adults waitlisted for kidney transplantation. Genet Med 23:1219–1224. https://doi.org/10.1038/s41436-021-01127-8
Devarajan P, Chertow GM, Susztak K, Levin A, Agarwal R, Stenvinkel P, Chapman AB, Warady BA (2022) Emerging role of clinical genetics in CKD. Kidney Med 4:100435. https://doi.org/10.1016/j.xkme.2022.100435
Mantan M, Batra V (2020) Renal biopsy in children. Indian Pediatr 57:452–458. https://doi.org/10.1007/s13312-020-1821-y
Domingo-Gallego A, Pybus M, Bullich G, Furlano M, Ejarque-Vila L, Lorente-Grandoso L, Ruiz P, Fraga G, López González M, Piñero-Fernández JA, Rodríguez-Peña L, Llano-Rivas I, Sáez R, Bujons-Tur A, Ariceta G, Guirado L, Torra R, Ars E (2022) Clinical utility of genetic testing in early-onset kidney disease: seven genes are the main players. Nephrol Dial Transplant 37:687–696. https://doi.org/10.1093/ndt/gfab019
Thomas CP, Freese ME, Ounda A, Jetton JG, Holida M, Noureddine L, Smith RJ (2020) Initial experience from a renal genetics clinic demonstrates a distinct role in patient management. Genet Med 22:1025–1035. https://doi.org/10.1038/s41436-020-0772-y
Gross O, Netzer KO, Lambrecht R, Seibold S, Weber M (2002) Meta-analysis of genotype-phenotype correlation in X-linked Alport syndrome: impact on clinical counselling. Nephrol Dial Transplant 17:1218–1227. https://doi.org/10.1093/ndt/17.7.1218
Kashtan CE (2022) Genetic testing and glomerular hematuria - a nephrologist’s perspective. Am J Med Genet C Semin Med Genet 190:399–403. https://doi.org/10.1002/ajmg.c.31987
Nevin SM, McLoone J, Wakefield CE, Kennedy SE, McCarthy HJ (2020) Genetic testing in the pediatric nephrology clinic: understanding families’ experiences. J Pediatr Genet 11:117–125. https://doi.org/10.1055/s-0040-1721439
Knoers N, Antignac C, Bergmann C, Dahan K, Giglio S, Heidet L, Lipska-Ziętkiewicz BS, Noris M, Remuzzi G, Vargas-Poussou R, Schaefer F (2022) Genetic testing in the diagnosis of chronic kidney disease: recommendations for clinical practice. Nephrol Dial Transplant 37:239–254. https://doi.org/10.1093/ndt/gfab218
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158:825–830. https://doi.org/10.7326/0003-4819-158-11-201306040-00007
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM, Subcommittee on screening and management of high blood pressure in children (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140:e20171904. https://doi.org/10.1542/peds.2017-1904
Gao M, Yu F, Dong R, Zhang K, Lv Y, Ma J, Wang D, Zhang H, Gai Z, Liu Y (2023) Diagnostic application of exome sequencing in Chinese children with suspected inherited kidney diseases. Front Genet 13:933636. https://doi.org/10.3389/fgene.2022.933636
Chen TK, Knicely DH, Grams ME (2019) Chronic kidney disease diagnosis and management: a review. JAMA 322:1294–1304. https://doi.org/10.1001/jama.2019.14745
Bassanese G, Wlodkowski T, Servais A, Heidet L, Roccatello D, Emma F, Levtchenko E, Ariceta G, Bacchetta J, Capasso G, Jankauskiene A, Miglinas M, Ferraro PM, Montini G, Oh J, Decramer S, Levart TK, Wetzels J, Cornelissen E, Devuyst O, Zurowska A, Pape L, Buescher A, Haffner D, Marcun Varda N, Ghiggeri GM, Remuzzi G, Konrad M, Longo G, Bockenhauer D, Awan A, Andersone I, Groothoff JW (2021) The European Rare Kidney Disease Registry (ERKReg): objectives, design and initial results. Orphanet J Rare Dis 16:251. https://doi.org/10.1186/s13023-021-01872-8
Köhler S, Gargano M, Matentzoglu N, Carmody LC, Lewis-Smith D, Vasilevsky NA, Danis D, Balagura G, Baynam G, Brower AM, Callahan TJ, Chute CG, Est JL, Galer PD, Ganesan S, Griese M, Haimel M, Pazmandi J, Hanauer M, Harris NL, Hartnett MJ, Hastreiter M, Hauck F, He Y, Jeske T, Kearney H, Kindle G, Klein C, Knoflach K, Krause R, Lagorce D, McMurry JA, Miller JA, Munoz-Torres MC, Peters RL, Rapp CK, Rath AM, Rind SA, Rosenberg AZ, Segal MM, Seidel MG, Smedley D, Talmy T, Thomas Y, Wiafe SA, Xian J, Yüksel Z, Helbig I, Mungall CJ, Haendel MA, Robinson PN (2021) The human phenotype ontology in 2021. Nucleic Acids Res 49:D1207–D1217. https://doi.org/10.1093/nar/gkaa1043
Best S, Lord J, Roche M, Watson CM, Poulter JA, Bevers RPJ, Stuckey A, Szymanska K, Ellingford JM, Carmichael J, Brittain H, Toomes C, Inglehearn C, Johnson CA, Wheway G, Genomics England Research Consortium (2022) Molecular diagnoses in the congenital malformations caused by ciliopathies cohort of the 100,000 Genomes Project. J Med Genet 59:737–747. https://doi.org/10.1136/jmedgenet-2021-108065
Riedhammer KM, Ćomić J, Tasic V, Putnik J, Abazi-Emini N, Paripovic A, Stajic N, Meitinger T, Nushi-Stavileci V, Berutti R, Braunisch MC, Hoefele J (2023) Exome sequencing in individuals with congenital anomalies of the kidney and urinary tract (CAKUT): a single-center experience. Eur J Hum Genet 31:674–680. https://doi.org/10.1038/s41431-023-01331-x
Author information
Authors and Affiliations
Contributions
All authors contributed to drafting, reviewing, and revising this paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to participate
Informed consent was obtained from legal guardians.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Caliment, A., Van Reeth, O., Hougardy, C. et al. A step-by-step, multidisciplinary strategy to maximize the yield of genetic testing in pediatric patients with chronic kidney diseases. Pediatr Nephrol (2024). https://doi.org/10.1007/s00467-024-06299-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00467-024-06299-4