Abstract
Background
IgA nephropathy (IgAN) is one of the most prevalent primary glomerulopathies in children. There are various studies investigating the efficacy of angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB) in adults with IgAN. However, only few studies evaluated the efficacy of these medications in pediatric patients.
Objective
To evaluate the efficacy and safety of ACEI/ARB in children with IgAN.
Data sources
Databases including PubMed, Web of Science, Cochrane, Scopus, and Google Scholar were searched between the 1st of April and 20th of July of 2021 using the keywords “IgA Nephropathy,” “Berger's Disease,” “Angiotensin-Converting Enzyme Inhibitors,” “Angiotensin Receptor Antagonists,” “Angiotensin II Type 1 Receptor Blockers,” and similar entry terms collected from the Medical Subject Headings (MeSH).
Study eligibility criteria
Observational studies (case series, case–control, cohort, and cross-sectional) and clinical trials with descriptions of pediatric patients (under 19 years old) with histopathological diagnosis of IgA nephropathy and who received ACEI and/or ARB.
Participants and interventions
Pediatric patients (under 19 years old) with histopathological diagnosis of IgA nephropathy and who received ACEI and/or ARB.
Study appraisal
For quality assessment, the Risk of Bias 2 tool (RoB 2), the Risk Of Bias In Non-randomized Studies of Interventions tool (ROBINS-I), the National Institutes of Health (NIH) quality assessment tool, and the Newcastle–Ottawa Scale (NOS) were used.
Results
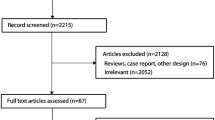
After recovering 1,471 studies, only eight, published between 2003 and 2019, met the eligibility criteria and were included in this systematic review. Of the 737 included children in the studies, 202 (25.8%) used ACEI/ARB and were compared with placebo and other therapy regimens. Of the seven studies that evaluated proteinuria, six reported an efficacy of ACEI/ARB in reducing this marker. ACEI/ARB also showed a possible effect in reducing hematuria and oxidative stress. The most common side effect was dizziness.
Limitations
The number of studies about the treatment with ACEI/ARB in children with IgAN is scarce. In addition, the studies are very heterogeneous. There are few studies that compared ACEI/ARB with placebo.
Conclusions and implications of key findings
The use of ACEI and/or ARB appears to be safe and to reduce proteinuria in pediatric patients with IgAN. Nonetheless, further randomized controlled trials, with greater methodological rigor and longer follow-up time, are required to establish the efficacy and safety of this therapy in this population.
Systematic review registration number
The protocol of this systematic literature review was registered in PROSPERO under the number CRD42021245375, and in the OSF registries (https://osf.io/qft4z/) with the registration https://doi.org/10.17605/OSF.IO/VADYR.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information


Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Cambier A, Boyer O, Deschenes G, Gleeson J, Couderc A, Hogan J et al (2020) Steroid therapy in children with IgA nephropathy. Pediatr Nephrol 35:359–366. https://doi.org/10.1007/s00467-018-4189-7
Lai KN (2012) Pathogenesis of IgA nephropathy. Nat Rev Nephrol 8:275–283. https://doi.org/10.1038/nrneph.2012.58
Coppo R (2021) Treatment of IgA nephropathy in children: a land without KDIGO guidance. Pediatr Nephrol 36:491–496. https://doi.org/10.1007/s00467-020-04486-7
Yata N, Nakanishi K, Shima Y, Togawa H, Obana M, Sako M et al (2008) Improved renal survival in Japanese children with IgA nephropathy. Pediatr Nephrol 23:905–912. https://doi.org/10.1007/s00467-007-0726-5
Selewski DT, Ambruzs JM, Appel GB, Bomback AS, Matar RB, Cai Y et al (2018) Clinical characteristics and treatment patterns of children and adults with IgA nephropathy or IgA vasculitis: findings from the CureGN study. Kidney Int Rep 3:1373–1384. https://doi.org/10.1016/j.ekir.2018.07.021
Berger J, Hinglais N (1968) Intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris) 74:694–695
Xie Y, Chen X, Nishi S, Narita I, Gejyo F (2004) Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int 65:1135–1144. https://doi.org/10.1111/j.1523-1755.2004.00486.x
Rasche FM, Schwarz A, Keller F (1999) Tonsillectomy does not prevent a progressive course in IgA nephropathy. Clin Nephrol 51:147–152
Coppo R, Roccatello D, Amore A, Quattrocchio G, Molino A, Gianoglio B et al (1990) Effects of a gluten-free diet in primary IgA nephropathy. Clin Nephrol 33:72–86
Coppo R (2017) Biomarkers and targeted new therapies for IgA nephropathy. Pediatr Nephrol 32:725–731. https://doi.org/10.1007/s00467-016-3390-9
Coppo R, Peruzzi L, Amore A, Piccoli A, Cochat P, Stone R et al (2007) IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol 18:1880–1888. https://doi.org/10.1681/ASN.2006040347
Ohashi N, Urushihara M, Kobori H (2009) Activated intrarenal reactive oxygen species and renin angiotensin system in IgA nephropathy. Minerva Urol Nefrol 61:55–66
Shima Y, Nakanishi K, Sako M, Saito-Oba M, Hamasaki Y, Hataya H et al (2019) Lisinopril versus lisinopril and losartan for mild childhood IgA nephropathy: a randomized controlled trial (JSKDC01 study). Pediatr Nephrol 34:837–846. https://doi.org/10.1007/s00467-018-4099-8
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Paez A (2017) Gray literature: an important resource in systematic reviews. J Evid-Based Med 10:233–240. https://doi.org/10.1111/jebm.12266
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
National Heart, Lung and Blood Institute (2014) Quality assessment tool for case series studies. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascularriskreduction/tools/case_series 2014. Accessed 20 July 2021.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 20 July 2021.
Yang Y, Ohta K, Shimizu M, Nakai A, Kasahara Y, Yachie A et al (2005) Treatment with low-dose angiotensin-converting enzyme inhibitor (ACEI) plus angiotensin II receptor blocker (ARB) in pediatric patients with IgA nephropathy. Clin Nephrol 64:35–40. https://doi.org/10.5414/cnp64035
Yagi K, Okada M, Yanagida H, Kuwajima H, Ikeda M, Sugimoto K et al (2003) Comparison of antiproteinuric effects of two different combination therapies in children with IgA nephropathy. Clin Exp Nephrol 7:270–274. https://doi.org/10.1007/s10157-003-0255-x
Tanaka H, Suzuki K, Nakahata T, Tsugawa K, Konno Y, Tsuruga K et al (2004) Combined therapy of enalapril and losartan attenuates histologic progression in immunoglobulin A nephropathy. Pediatr Int 46:576–579. https://doi.org/10.1111/j.1442-200x.2004.01955.x
Nakanishi K, Iijima K, Ishikura K, Hataya H, Awazu M, Sako M et al (2009) Efficacy and safety of lisinopril for mild childhood IgA nephropathy: a pilot study. Pediatr Nephrol 24:845–849. https://doi.org/10.1007/s00467-008-1006-8
Pei Y, Xu Y, Ruan J, Rong L, Jiang M, Mo Y et al (2016) Plasma oxidative stress level of IgA nephropathy in children and the effect of early intervention with angiotensin-converting enzyme inhibitors. J Renin Angiotensin Aldosterone Syst 17:1470320316647240. https://doi.org/10.1177/1470320316647240
Foster BJ, Bernard C, Drummond KN, Sharma AK (2000) Effective therapy for severe Henoch-Schonlein purpura nephritis with prednisone and azathioprine: a clinical and histopathologic study. J Pediatr 136:370–375. https://doi.org/10.1067/mpd.2000.103448
Katafuchi R, Kiyoshi Y, Oh Y, Uesugi N, Ikeda K, Yanase T et al (1998) Glomerular score as a prognosticator in IgA nephropathy: its usefulness and limitation. Clin Nephrol 49:1–8
Cheng J, Zhang W, Zhang XH, He Q, Tao XJ, Chen JH (2009) ACEI/ARB therapy for IgA nephropathy: a meta analysis of randomised controlled trials. Int J Clin Pract 63:880–888. https://doi.org/10.1111/j.1742-1241.2009.02038.x
Suzuki Y, Matsuzaki K, Suzuki H, Okazaki K, Yanagawa H, Ieiri N, Sato M, Sato T, Taguma Y, Matsuoka J, Horikoshi S, Novak J, Hotta O, Tomino Y (2014) Serum levels of galactose-deficient immunoglobulin (Ig) A1 and related immune complex are associated with disease activity of IgA nephropathy. Clin Exp Nephrol 18:770–777. https://doi.org/10.1007/s10157-013-0921-6
Cheng J, Zhang X, Tian J, Li Q, Chen J (2012) Combination therapy an ACE inhibitor and an angiotensin receptor blocker for IgA nephropathy: a meta-analysis. Int J Clin Pract 66:917–923. https://doi.org/10.1111/j.1742-1241.2012.02970.x
Arakawa K (1996) Serine protease angiotensin II systems. J Hypertens Suppl 14:S3–S7
Pagano PJ, Ito Y, Tornheim K, Gallop PM, Tauber AI, Cohen RA (1995) An NADPH oxidase superoxide-generating system in the rabbit aorta. Am J Physiol 268:H2274–H2280. https://doi.org/10.1152/ajpheart.1995.268.6.H2274
Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, Suzaki Y, Shoji T (2007) Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun 358:156–163. https://doi.org/10.1016/j.bbrc.2007.04.105
Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Urushihara M, Kobori H (2009) Role of activated intrarenal reactive oxygen species and renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol 36:750–755. https://doi.org/10.1111/j.1440-1681.2009.05172.x
Izzo JL Jr, Weir MR (2011) Angiotensin-converting enzyme inhibitors. J Clin Hypertens (Greenwich) 13:667–675. https://doi.org/10.1111/j.1751-7176.2011.00508.x
Li PK, Leung CB, Chow KM, Cheng YL, Fung SK, Mak SK, Tang AW, Wong TY, Yung CY, Yung JC, Yu AW, Szeto CC, HKVIN Study Group (2006) Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis 47:751–760. https://doi.org/10.1053/j.ajkd.2006.01.017
Kifor I, Moore TJ, Fallo F, Sperling E, Chiou CY, Menachery A, Williams GH (1991) Potassium-stimulated angiotensin release from superfused adrenal capsules and enzymatically dispersed cells of the zona glomerulosa. Endocrinology 129:823–831. https://doi.org/10.1210/endo-129-2-823
Bakris GL, Weir MR (2000) Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med 160:685–693. https://doi.org/10.1001/archinte.160.5.685
Funding
This work was partially supported by Brazilian National Council of Research Development (CNPq—Grant # 302153/2019-5), Coordination of High Education Level Personnel (CAPES), and Foundation of Research of Minas Gerais (FAPEMIG).
Author information
Authors and Affiliations
Contributions
PASVC, LB, BWSP, AQRL, HSH, CRMN, and MDM registered the study in PROPERO and in Open Science Framework. PASVC, LB, BWSP, AQRL, HSH, CRMN, and MDM made the literature revision and selection of the articles to be included. BWSP, AQRL, HSH, CRMN, and MDM extracted data from the selected articles. PASVC and LB checked data extraction and evaluated the quality of selected articles. PASVC, LB, BWSP, AQRL, HSH, CRMN, and MDM wrote the first draft. ACSS conceptualized the study, made general supervision, and revised the manuscript. ACSS submitted the final version of the manuscript, which is approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vaz de Castro, P.A.S., Bitencourt, L., Pereira, B.W.S. et al. Efficacy and safety of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for IgA nephropathy in children. Pediatr Nephrol 37, 499–508 (2022). https://doi.org/10.1007/s00467-021-05316-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05316-0